The modulation of the recognition site is of great significance to the development of highly selective and sensitive fluorescent sensors, and the promotion of response speed for various on-site applications.
However, it has rarely been focused on the crucial thermodynamic factor related to the preferential combination between the recognition site and the target molecules neither from the aspect of experimental investigation nor theoretical simulation.
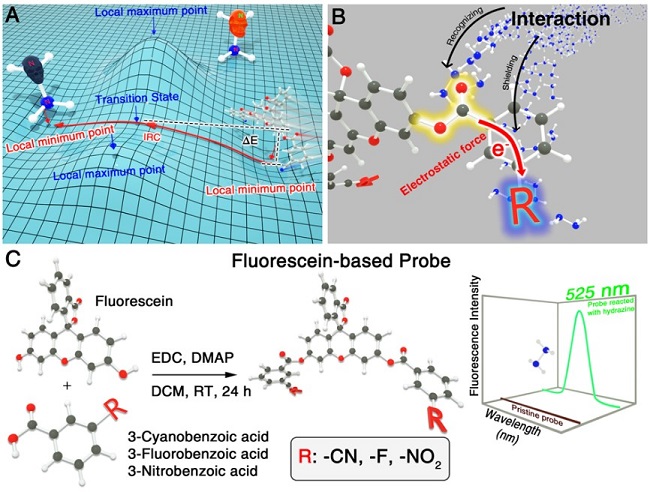
The research group led by Prof. DOU Xincun from the Xinjiang Technical Institute of Physics & Chemistry of the Chinese Academy of Sciences (CAS) has proposed a highly efficient respond speed accelerating strategy for the on-site detection of the hazardous hydrazine (N2H4) through precisely modulating the non-covalent interaction between the ester bond and hydrazine.
This study was published in Cell Reports Physical Science on May 2.
The modulation strategies of the recognition site on the trace hydrazine detecting fluorescent probes (Image by LI Jiguang)
The fluorescein was esterified with 3-cyanobenzoic acid, 3-fluorobenzoic acid and 3-nitrobenzoic acid respectively, and thus three structurally similar intramolecular charge transfer (ICT) fluorescein-based probes (F-CN, F-F and F-NO2) were obtained through the change of R-benzene groups (R=-CN, -F and -NO2).
For the purpose of boosting the response speed of fluorescent probe in chemical reaction system, the thermodynamic force of the recognition site was regulated considering the influence from the electrostatic effect and the shielding effect of the R-benzene group adjacent to the recognition site.
Through quantum chemical and molecular dynamic analysis, it was found that the designed fluorescent probe enables a high electrostatic potential for the ester bond to strongly combine with the target electrostatically negative hydrazine (-35.96 kcal/mol), as well as a moderate shielding effect and a favorable inverse Gibbs free energy barrier, thus, ensuring the effective combination with hydrazine to boost the response speed.
The strong electrostatic combine capability, the moderate shielding effect and the favorable inverse Gibbs free energy barrier, together ensured the F-CN probe to possess the most effective combination and reaction with hydrazine with a LOD as low as 2 nM (64 ppt) and a boosted light up time of 2-3 s, which is much more superior than the previously reported fluorescent probes which generally need 600-3600 s to respond.
Furthermore, the applicability of the proposed F-CN probe for the rapid, on-site and reliable detection of hydrazine was demonstrated by the exploration of a portable fluorescence detector with promising utilization.
“The present investigation would facilitate the accurate exploration of fluorescent probes for ultrasensitive and rapid chemical sensing, as well as the establishment of non-covalent interaction investigation for various applications,” said Prof. DOU.
