A New Supercapacitor’s Electrode Material with High Specific Capacitance and Low Cost Prepared
Editor: | Jun 28,2013
Supercapacitor is the most promising electrochemical energy storage device due to its higher power density and longer cycle life compared with the secondary battery. It has been widely applied for the information technology industry, such as electronic devices, electric vehicles, etc.
The electrode is one of the most important components for a supercapacitor. As the common materials of electric double-layer capacitor (EDLC) electrodes, there are metal oxides, polymers, and porous materials and so on. Besides, new carbon materials, like carbon nanotube, have also been developed as a electrode material, but there are difficulties in practical application because of their complicated preparation and high cost.
Researchers from Xinjiang Technical Institute of Physics & Chemistry, Chinese Academy of Sciences (XTIPC), prepared a new activated carbon with high specific capacitance and low cost,employing cotton stalk as the raw material, by using the phosphoric acid (H3PO4) chemical activation methodat a certain temperature.
Among different ratios of cotton stalk to activating agent, researchers found H3PO4-to-carbon mass impregnation ratio of 1:4, the specific capacitance is largest (Fig1). Based on Brunauer–Emmett–Teller (BET) and t-plot results,the specific capacitance of activated carbon from cotton stalk in organic electrolyte is up to 98 F g?1 at 2 A g-1 , and keeps remarkably stable over 500 cycles. Which shows the high capacitance retention ratio is 95.3%(Fig.2). These results demonstrate that the activated carbons derived from cotton stalk exhibit well cycle stability and very high degree of reversibility during repetitive charge/discharge cycles. Because of the unique characteristic, the simple preparation, and the large availability of material, the cotton stalk will become one kind of potential material for supercapacitor electrode.
The study was published in Journal of Solid State Electrochemistry (April 2013, Volume 17, Issue 4, pp 1005-1012).
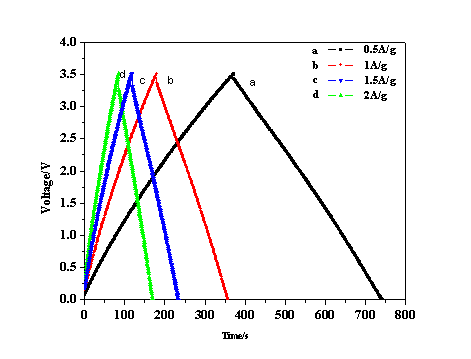
Fig. 1 The charge/discharge curves of activated carbon at different current density

Fig. 2 Charge-discharge cycling stability of activated carbon at a current density of 2 A g?1
附件下载:
 (86) 991-3838931
(86) 991-3838931 lhskj@ms.xjb.ac.cn
lhskj@ms.xjb.ac.cn (86)991-3838957
(86)991-3838957 40-1 Beijing Road
Urumqi, XinjiangChina
40-1 Beijing Road
Urumqi, XinjiangChina