Cysteine-modified Orange Peel for Removal of Cu(II) from Aqueous Solutions
Editor: | Jul 19,2013
Copper is widely applied to the electrical and electroplating industry, exhibits high acute and chronic toxicity. Due to Cu(II) is not biodegradable like many organic pollutants, its removal is important in environmental remediation and clean-up efforts. Adsorption possesses advantages such as high efficiency in treating aqueous solutions with relatively low metal ion concentrations and producing suitable effluent for reuse without secondary pollution. Removal of heavy metal ions with adsorbent gained from low-cost and renewable biomass or agriculture wastes is of particularly interesting.
Researchers at Xinjiang Technology Institute of Physics & Chemistry, Chinese Academy of Sciences (XTIPC), employed cysteine-modified orange peel (COP) for the removal of Cu(II) from aqueous solutions has been developed and comparatively studied with diethylenetriamine-modified orange peel (DOP). Researchers systematically evaluated COP and DOP by their capabilities for adsorbing Cu(II), including the key influential parameters such as initial pH, contact time and initial Cu(II) concentration.
The experiment results show that the maximum adsorption capacities of Cu(II) onto COP and DOP are 95.23 and 83.68 mg/g, respectively(Table1). The recycle experiment shows that the developed adsorbent can be successfully used for five consecutive adsorption–desorption cycles with only a marginal loss in the adsorption efficiency. Therefore, the work suggests the possibility of applying orange peel to the removal and recycle of Cu(II) from wastewater.
The result has been published in Water Science and Technology, 2013, 67(11): 2444-2450.
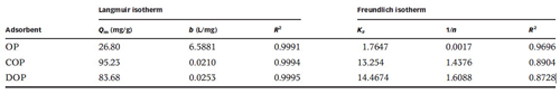
Table1 Langmuir and Freundlich isotherms constants for Cu(II) adsorption on OP, COP and DOP
附件下载:
 (86) 991-3838931
(86) 991-3838931 lhskj@ms.xjb.ac.cn
lhskj@ms.xjb.ac.cn (86)991-3838957
(86)991-3838957 40-1 Beijing Road
Urumqi, XinjiangChina
40-1 Beijing Road
Urumqi, XinjiangChina