Two New Borate Halides Designed and Synthesized
Editor: | Aug 23,2013
In recent years, nonlinear optical (NLO) crystals become academic and commercial interest attributable to their prominent applications in the laser science and optoelectronic devices. However, the basic requirement for NLO materials is that they must crystallize in the noncentrosymmetric (NCS) space group.
Prof. PAN Shilie’s group at Xinjiang Technical Institute of Physics & Chemistry, Chinese Academy of Sciences(XTIPC), has been obtained some good NLO materials with wide transparence window and large second harmonic generation (SHG) response by introducing the halogen atoms into borates. Recently, the group successfully synthesized a new deep UV NLO crystal Cs2B4SiO9, with the large SHG response and deep UV cutoff edge.
Based on the previous research, Prof. PAN Shilie’s group introduced a new rigid unit, AlO4 tetrahedron, into B–O framework to design a [AlB4O9]∞ framework similar to the [SiB4O10]∞framework, whic h is responsible for the large SHG response and short UV cutoff edge of Cs2B4SiO9. Besides, to better understand the effect of the rigid units on the symmetry of the framework, centrosymmetric NaBa4(B5O9)2F2Cl are also synthesized by using B atoms to substitute Al atoms. Researchers explained the basic building units of NaBa4(AlB4O9)2Br3 are the isolated AlO4 tetrahedra and B4O9 groups, which are composed of two BO3 triangles and two BO4 tetrahedra by sharing O atoms. Each B4O9 group is linked to four different AlO4 tetrahedra through its terminal O atoms, and likewise each AlO4 tetrahedron shares its four vertices with four neighboring B4O9 groups to form 3D [AlB4O9] backbone. The Na, Ba, and Br atoms are filled in the cavities of [AlB4O9] backbone (Fig.1).
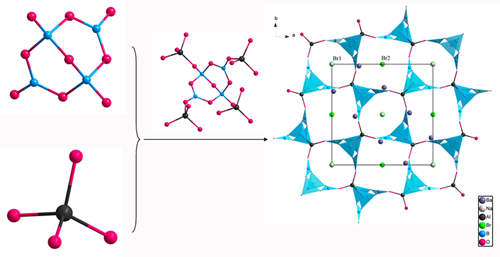
Fig.1 The structure of NaBa4(AlB4O9)2Br3
Researchers found NaBa4(AlB4O9)2Br3 and NaBa4(B5O9)2F2Cl have similar stoichiometry, they exhibit obviously different structures. NaBa4(AlB4O9)2Br3 is NCS and polar, while NaBa4(B5O9)2F2Cl is centrosymmetric. The structural analyses show that the difference is mainly due to the rigid AlO4 tetrahedra reducing the degree of freedom of the connection of the building units and increasing the distortion of B–O groups in framework.
The result has been published on Crystal Growth Design, 2013, 13 (8), pp 3514–3521.
附件下载:
 (86) 991-3838931
(86) 991-3838931 lhskj@ms.xjb.ac.cn
lhskj@ms.xjb.ac.cn (86)991-3838957
(86)991-3838957 40-1 Beijing Road
Urumqi, XinjiangChina
40-1 Beijing Road
Urumqi, XinjiangChina