Influence of the Na+ Substitution on Structural Transition and Second Harmonic Generation Property
Editor: | Apr 09,2014
In crystal engineering, modification of the crystal structure by cation substitution is one of the most important and efficient methods to obtain new structures with different properties. It is well known that cations with similar ionic radius or coordinate environment are easily to replace each other without changing the host crystal structures.
In order to design and discover new nonlinear optical(NLO)crystals, approaches by cations substitution are often adopted. A group of researchers lead by professor PAN Shilie from Xinjiang Technical Institute of Physics & Chemistry, Chinese Academy of Sciences (XTIPC), designed and synthesized four stoichiometric crystals, K3-xNaxB6O10 (x=0.13, 0.67, 1.30, 2.20), by introducing Na cations into K3B6O10Br. Interestingly, they have the similar formula type of K3-xNaxB6O10Br, however, they crystallize in a different space group, R3m and Pnma for K3-xNaxB6O10Br (x=0.13, 0.67) and K3-xNaxB6O10Br (x=1.30, 2.20), respectively (Fig.1). The second harmonic generation (SHG) response of K3-xNaxB6O10Br (x=0.13, 0.67) is about 2.8 times of that of KDP (Fig.2).
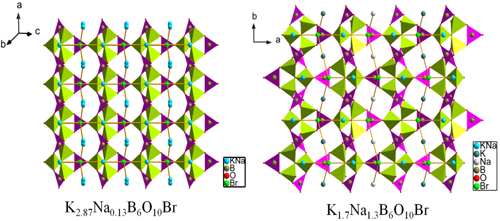
Fig.1 The 3D framework of K3-xNaxB6O10Br (x=0.13, 1.3)
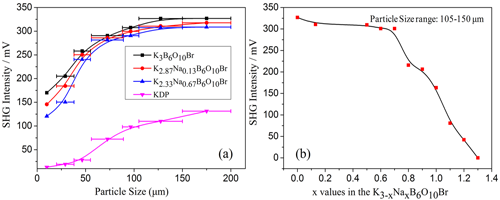
Fig.2 Phase-matching, i.e., particle size vs. SHG intensity, datas for K2.87Na0.13B6O10Br, K2.33Na0.67B6O10Br, K3B6O10Br and KDP
Detailed structural analyses suggest that the coordination environments of cations play critical roles to determine the symmetry of the Br-M (M=K/Na, K, or Na) lattices, which influences the overall structure of the crystal.
The research results have been published in the Cryst. Growth Des., 2014.This work is supported by the “National Natural Science Foundation of China”, the High-level Professional and Technical Personnel of Autonomous region, etc.
附件下载:
 (86) 991-3838931
(86) 991-3838931 lhskj@ms.xjb.ac.cn
lhskj@ms.xjb.ac.cn (86)991-3838957
(86)991-3838957 40-1 Beijing Road
Urumqi, XinjiangChina
40-1 Beijing Road
Urumqi, XinjiangChina