Scientists Synthesize Pb2Ba3(BO3)3Cl with Large SHG Enhancement Activated by Pb-Chelated BO3 Groups
Editor: | Aug 26,2015
Nowadays, a variety of commercial new nonlinear optical (NLO) crystals have been developed. Pb(II) is often used to substitute alkaline earth metal in borates to create NLO materials with excellent second harmonic generation (SHG) response due to the lone pair activity. However, large enhancement is rare by replacing the alkaline earth metal with Pb(II) in Pb-free borates and the mechanism of the SHG enhancement is still a mystery.
A research group led by Prof. PAN Shilie and Prof. YANG Zhihua at Xinjiang Technical Institute of Physics & Chemistry of Chinese Academy of Sciences, in collaboration with American scientists from Northwestern University clarified the reason of the SHG enhancement, and based on the result, they designed and synthesized a new NLO material Pb2Ba3(BO3)3Cl for the first time. The obtained Pb2Ba3(BO3)3Cl shows a phase-matching SHG response approximately 3.2 times that of KDP, and as large as 6 times that of isomorphic Ba5(BO3)3Cl, which is the first acentric lead borate with isolated BO3 groups.
Motivated by the large SHG response obtained from the properties of isolated boron?oxygen groups and lead?oxygen groups, researchers designed and synthesized the acentric compound Pb2Ba3(BO3)3Cl with isolated BO3 groups. To further study the relationship between the SHG response and the electronic structure, they used the first principle calculation and discovered that the enhanced response originates from the unique edge sharing connection style between the Pb?O polyhedra and B?O groups.
This unique connection style enhances SHG responses by making the metal?oxygen bond more polarizable to external optical excitation. Therefore, researchers conjectured that among lead A-L replacement borates, those compounds with isolated boron?oxygen building units would be potential NLO materials with enhanced SHG responses and merit continued investigation.
The study, which is published in Journal of the American Chemical Society , provides a route to identify systems that would benefit from SHG-active cation substitution in isomorphic structures that exhibit weak or null SHG responses.
This work was financially supported by the National Basic Research Program of China, National Natural Science Foundation of China, the Western Light of Chinese Academy of Sciences, the Recruitment Program of Global Experts, etc.
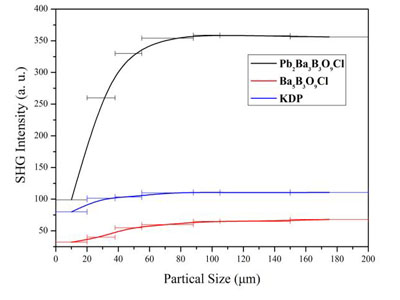
Figure 1:Phase-matching curves of Pb2Ba3(BO3)3Cl, Ba5(BO3)3Cl, and KDP (Image by XTIPC)
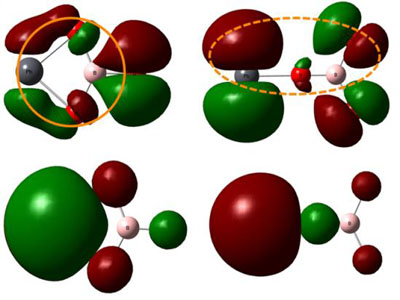
Figure 2: Diagram of the structures and the frontier molecular orbitals (isovalue 0.02 au/?3) of PbBO3 (Image by XTIPC)
Contact:
Prof. PAN Shilie
E-mail:slpan@ms.xjb.ac.cn
Xinjiang Technical Institute of Physics & Chemistry,CAS
附件下载:
 (86) 991-3838931
(86) 991-3838931 lhskj@ms.xjb.ac.cn
lhskj@ms.xjb.ac.cn (86)991-3838957
(86)991-3838957 40-1 Beijing Road
Urumqi, XinjiangChina
40-1 Beijing Road
Urumqi, XinjiangChina