Researchers Reveal Influence Mechanism of Surface Defects on the Photocatalytic Activity of Photocatalysts
Editor: | Mar 30,2016
TiO2 is a frequently studied photocatalyst due to its excellent physicochemical properties as well as its earth abundance, nontoxicity and stability. However, the large band gap limits its utilization of sunlight and practical applications. Besides TiO2, graphitic carbon nitride (g-C3N4) is another promising photocatalyst because of its high thermal and chemical stability, semiconductivity and desirable band gap of 2.7 eV. Unfortunately, the visible light photocatalytic activity of g-C3N4 is still low because of the fast recombination of the photogenerated charge carriers.Therefore, it is essential to design and develop new strategies which could efficiently improve the photocatalytic activity of TiO2 and g-C3N4.
Surface defects are very important in the process of photocatalysis. They play an important role in modifying the electronic structure and surface structure and consequently the photocatalytic activity of semiconductor photocatalysts. A team of researchers led by WANG Chuanyi at Xinjiang Technical Institute of Physics & Chemistry of Chinese Academy of Sciences (XTIPC) demonstrated that the photocatalytic activity of TiO2 and g-C3N4 could be well turned by the introduction of surface defects.
By controlling the amount of L-ascorbic acid in the precursor of TiO2, researchers introduced oxygen vacancies (OVs) in the framework of TiO2, and it was found that the defective TiO2-x showed high photocatalytic efficiency in pollutants degradation and in hydrogen evolution under visible light (Figure 1). The cycling test of TiO2?x for hydrogen evolution indicated the good stability of defective TiO2?x under the visible light irradiation.
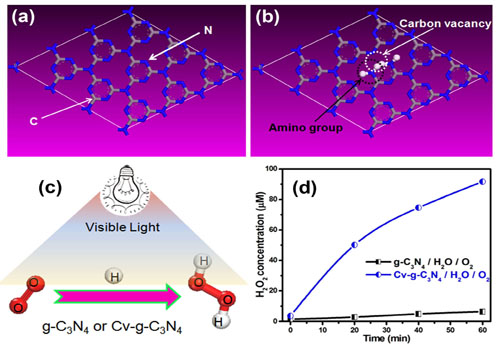
Nitrogen vacancies (NVs) and carbon vacancies (CVs) were respectively introduced on the surface of a metal-free polymer semiconductor, graphitic carbon nitride (g-C3N4). It was interesting to notice that NVs could endow g-C3N4- with the photocatalytic N2 fixation ability (Figure 2). The Photocatalytic N2 fixation induced by NVs is free from the interference of other gases. Additionally, CVs greatly improved the activity of g-C3N4 for H2O2 production in the absence of organic scavenger (Figure 3). CVs could change the H2O2 generation pathway from a two-step single-electron indirect reduction to a one-step two-electron direct reduction.
Researchers designed a series of experiments to identify the roles of surface defects in the process of photocatalysis. The presence of surface defects could reduce the symmetry of photocatalysts and narrow down its band gap, thus extending the visible light absorption and increasing the excitable electrons. Besides, surface defects have been found to be able to trap photogenerated electrons, thus inhibiting the recombination of photogenerated electrons and holes. Meanwhile, they can also enhance the adsorption and activation of gaseous molecules because of their abundant localized electrons.
The related results were published online in Applied Catalysis B: Environmental and Journal of Materials Chemistry A.
The work was supported by the National Science Foundation of China, “Western Light” Program of CAS, and the CAS/SAFEA International Partnership Program for Creative Research Teams.
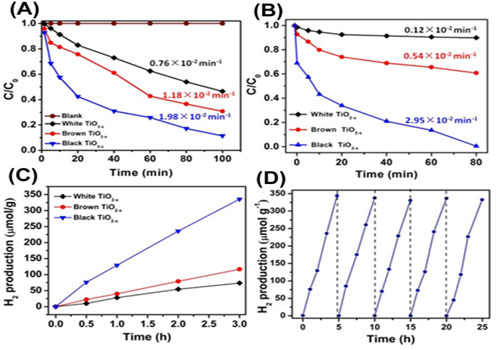
Figure 1. Degradation of organic pollutions and cycle test of the defective TiO2-x nanocrystals.(Image by XTIPC )
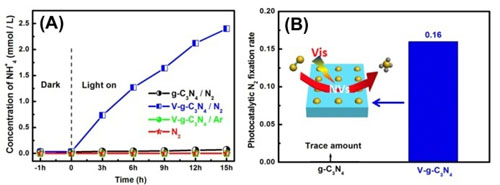
Figure 2. Photocatalytic N2 fixation on g-C3N4 and V-g-C3N4.(Image by XTIPC)
Contact:
Prof.WANG Chuanyi; Dr.DONG Guohui
E-mail:cywang@ms.xjb.ac.cn;donggh@ms.xjb.ac.cn
Xinjiang Technical Institute of Physics & Chemistry,CAS
附件下载:
 (86) 991-3838931
(86) 991-3838931 lhskj@ms.xjb.ac.cn
lhskj@ms.xjb.ac.cn (86)991-3838957
(86)991-3838957 40-1 Beijing Road
Urumqi, XinjiangChina
40-1 Beijing Road
Urumqi, XinjiangChina