Researchers Develop Z-scheme Heterojunction Photocatalyst for NO Removal
Editor: | Sep 08,2016
NO is a common gaseous pollutant which can cause environmental and health problems, such as acid rain, photochemical smog, haze, and even pulmonary edema. The concentration of NO in the atmosphere has greatly increased over the past few decades due to the increased automobiles and industrial activities. Although some methods including thermal catalysis reduction and physical or chemical adsorption have been adopted to remove NO from atmosphere, most of them are low efficient and may produce secondary pollution.
Semiconductor photocatalytic technology has been recently regarded as an attractive alternative technology for NO removal. Among various photocatalysts, g-C3N4 is a metal-free polymeric semiconductor material, which becomes increasingly promising for photocatalysis under visible light, mainly due to its desirable band gap of 2.7 eV, strong robustness and low cost.
A research group led by Prof. WANG Chuanyi at Xinjiang Technical Institute of Physics & Chemistry(XTIPC) of Chinese Academy of Sciences constructed an all-solid-state Z-scheme heterojunction (PI-g-C3N4) consisting of g-C3N4 surface-modified with perylene imides (PI).
In fact, photosynthesis with Z-scheme charge transfer process can separate the photogenerated electrons and holes into two photosystems through the electron mediator. Inspired by the advantage of photosynthesis, researchers developed an all-solid-state Z-Scheme heterojunction (PI-g-C3N4). As tested for photocatalytic removal of NO under visible light, significant enhancement in catalytic activity was observed for PI-g-C3N4 in comparison with its counterpart, the pristine g-C3N4.
Researchers found that the Z-scheme heterojunction creates charge separation with electron populated to the higher conduction band and hole to the lower valence band, thus enhancing the redox reaction power of the charge carriers. The separated hole can directly oxidize NO to NO2, while the electron can directly reduce O2 to H2O2. NO2 and H2O2 can react at different sites (via diffusion), and the produced NO3- ion would cause minimal deactivation to the catalyst (see Figure).
This study provides new insight into the design of effective visible light-driven photocatalysts, which improves the utilization of solar energy.
The work was supported by the National Science Foundation of China, the CAS/SAFEA International Partnership Program for Creative Research Teams, “Western Light” Program of Chinese Academy of Sciences, etc.
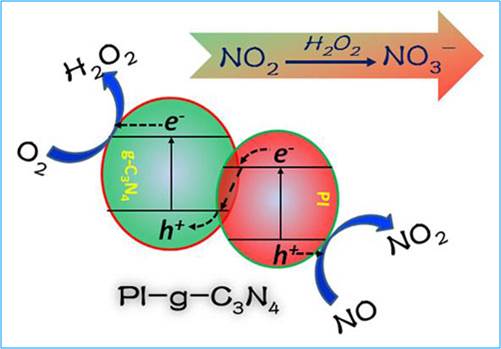
Figure: NO photocatalytic mechanism of PI-g-C3N4 under visible light irradiation.(Image by DONG Guohui)
Contact:
Prof.WANG Chuanyi
E-mail: cywang@ms.xjb.ac.cn
Xinjiang Technical Institute of Physics & Chemistry,CAS
附件下载:
 (86) 991-3838931
(86) 991-3838931 lhskj@ms.xjb.ac.cn
lhskj@ms.xjb.ac.cn (86)991-3838957
(86)991-3838957 40-1 Beijing Road
Urumqi, XinjiangChina
40-1 Beijing Road
Urumqi, XinjiangChina