Research Progress in Selective Oil/Water Separation Materials
Editor: | Dec 27,2023
The oil/wateremulsion is a colloid system consisting of oil (or other nonpolar liquid) dispersed with an emulsifier in water. The efficient separation of emulsion has become a difficult problem because of its high stability and small oil droplet sizes. Therefore, developing novel membranes for effectively dealing with oil/water emulsion is urgently necessary. The critical step in the emulsion separation process is demulsification through the membranes. The mechanism of demulsification by membrane-emulsion interaction have been widely concerned by researchers. Most of the materials reported could realize the separation of one kind of specific emulsion. The effect ofmembrane surface charge on the demulsification progress needs to be further studied.
Recently, the Separation Materials and Technology research group of Xinjiang Technical Institute of Physics and Chemistry developed a simple and efficient method to fabricate the polyethylenimine (PEI) modified glass fibers (GF) membrane by the sulfur-based coupling reaction and wet-laid process. The membranes before and after the grafting of PEI both showed superamphiphilicity in air and underwater superoleophobicity. While, surface modification by PEI endowed the PEI-GF membrane with a positively charged surface (+17.3 mV). The prepared membranes can effectively induce the demulsification and separation of O/W emulsions stabilized by anionic (sodium dodecyl sulfate, SDS), nonionic (polyethylene glycol sorbitan monooleate, Tween 80), and zwitterionic (dodecyl betaine, BS-12) surfactants. Especially, the separation efficiency of the PEI-GF membrane to SDS stabilized emulsions significantly enhanced to 95.4%. However, the separation efficiency was only 31.8% for the emulsion stabilized by a cationic surfactant (hexadecyltrimethylammonium bromide, CTAB). Based on the above results, the mechanism of selective separation of the emulsion has been proposed. The positive charges of the PEI-GF membrane were caused by protonation of abundant amino groups of PEI. When the PEI-GF membrane was in contact with emulsions, the protonated PEI segment stretched toward the water phase of the emulsion and was absorbed on the oil/water interfacial film via electrostatic interaction or hydrogen bonding, evoking a decrease in the interfacial film stability and demulsification. Consequently, the small droplets aggregated with each other to form larger ones and the oil droplets were finally floated from the fiber surface under buoyancy (Fig. 1). On the contrary, there was strong electrostatic repulsion between the CTAB stabilized emulsion and the PEI-GF membrane, which was unfavorable for the destruction of the oil/water interfacial film. This mechanism has been verified in the treatment of emulsified oily wastewater of petrochemical industry, and has been applied to wastewater engineered treatment unit with capacity 500 m3/day.
The research results were published in the ACS Applied Polymer Materials. The research work is supported by the project of the West Light Foundation of CAS and Natural Science Foundation of Xinjiang Uygur Autonomous Region.
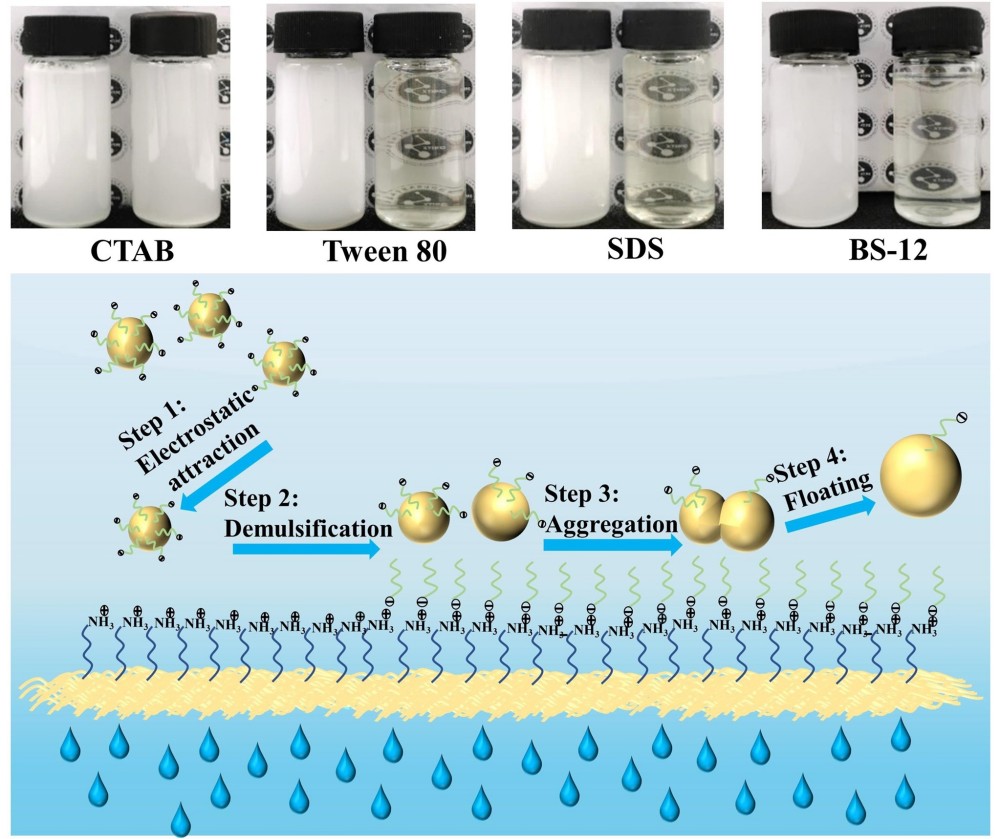
Fig. 1 Schematic diagram of electrostatic/hydrogen bonding assisted aggregation induces demulsified oil-water separation
附件下载:
 (86) 991-3838931
(86) 991-3838931 lhskj@ms.xjb.ac.cn
lhskj@ms.xjb.ac.cn (86)991-3838957
(86)991-3838957 40-1 Beijing Road
Urumqi, XinjiangChina
40-1 Beijing Road
Urumqi, XinjiangChina