Chinese Scientists Unveil Molecular Engineering Pore Structure for Sodium-Ion Storage of Hard Carbon Anodes
Editor: YIN Jiao | Feb 26,2025
Sodium-ion batteries are the most promising solution for low-cost, short- and medium-term, large-scale energy storage needs, but their practical applications are restricted by the high manufacturing cost, low capacity, low initial coulombic efficiency, and slow kinetics of hard carbon anodes. The research and development of hard carbons focus on overcoming the following issues: The active structures for Na-ion storage are unclear, which restricts the structural designs of hard carbons. On the other hand, there lack of hard carbon materials of low costs, high-sodium storage capacities, and scalable preparation.
A recent study by Chinese scientists has made significant strides in the development of high-performance sodium-ion batteries (SIBs) by focusing on the molecular engineering of hard carbon anodes. Led by researchers from the Xinjiang Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, the study provides new insights into optimizing the pore structure and interfacial functional groups of hard carbon, a promising anode material for SIBs.
The findings were published in Energy Storage Materials in an article titled "Molecular engineering of pore structure/interfacial functional groups toward hard carbon anode in sodium-ion batteries - ScienceDirect" on February, 2025.
The research not only deepens the understanding of the sodium storage mechanisms in hard carbon but also offers practical strategies for enhancing the performance of SIBs, which are considered a viable alternative to lithium-ion batteries due to the abundance and low cost of sodium resources.
Hard carbon, composed of disordered stacked graphitic carbon and turbine-like nanodomains, is a competitive anode material for SIBs due to its low operating voltage, simple synthesis process, and abundant resources. However, achieving a balance between high reversible capacity and initial coulombic efficiency (ICE) has been a significant challenge. The study addresses this by introducing different heteroatoms into the precursor to regulate the pore structure and optimize the interface of hard carbon.
The researchers designed three types of heteroatom-doped hard carbons by pyrolyzing precursors crosslinked with sucrose and dopants containing oxygen (O), nitrogen (N), and sulfur (S), respectively. The oxygen-doped hard carbon (HC-O) exhibited the best sodium storage performance, with a high reversible capacity of 352.9 mAh/g and an ICE of 88.0%. This performance is attributed to the abundant micropores (0.5–0.9 nm) and carbonyl groups (C=O) on the surface, which enhance both slope and plateau capacities and promote the formation of a uniform, inorganic-rich solid electrolyte interface (SEI).
The study systematically investigated the influence of different heteroatom cross-linked precursors on hard carbon modification. The results showed that the oxygen-containing dopants significantly improved the sodium storage performance by increasing active sites and expanding the carbon layer spacing, which facilitates the pseudocapacitive adsorption of sodium ions. Additionally, the abundant C=O groups on the surface of HC-O induced the formation of a thin and uniform SEI, improving ICE and cycling stability.
This research provides new insights into the design of high-performance hard carbon anodes for SIBs, offering a pathway to improve the energy density and cycling stability of sodium-ion batteries. The findings could pave the way for the development of more efficient and cost-effective energy storage systems, contributing to the advancement of renewable energy technologies.
The study was funded by the the National Natural Science Foundation of China, the Xinjiang Uygur Autonomous Region, the Youth Innovation Promotion Association of the Chinese Academy of Sciences.
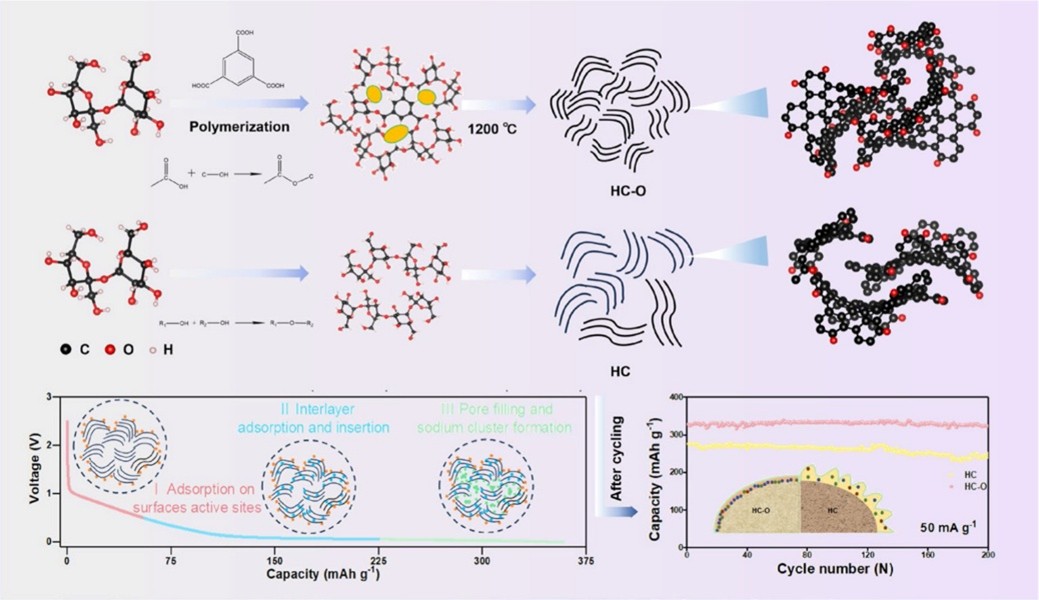
Figure 1. Heteroatom pyrolysis strategy for the closed pores of sodium-ion storage. (lmaged by YIN Jiao's Group)
附件下载:
 (86) 991-3838931
(86) 991-3838931 lhskj@ms.xjb.ac.cn
lhskj@ms.xjb.ac.cn (86)991-3838957
(86)991-3838957 40-1 Beijing Road
Urumqi, XinjiangChina
40-1 Beijing Road
Urumqi, XinjiangChina