Prismatic Crystal Field Stabilized Divalent Rare-Earth Chalcogenide REIIBII6CIII6QVI16 with Bifunctionality
Editor: LI Junjie | Mar 10,2025
Rare earth (RE) compounds exhibit unique electronic configurations and optoelectronic properties. They have received considerable attentions for decades and shown wide applications in nonliner optic, luminescence materials, biological therapy, catalysis, etc. Compared to RE3+, RE2+ ions show distingushed electronic configurations, as well as unusual catalytic, magnetic and optical properties, which is a hot topic in luminescence materials and RE chemistry. However, the synthesis of stable RE2+ compounds is still tardy because of the chemical instability of RE2+ ions, and the known RE2+ compounds like monooxides YO and LaO, or monochalcogenides LaS and SmS are usually fabricated in the form of epitaxial thin films induced by heterogeneous-substrates, limiting the development of RE chemistry and RE2+ based inorganic photoelectric functional materials .
To address this challenge, a research team led by Prof. Pan Shilie from the Xinjiang Technical Institute of Physics and Chemistry of the Chinese Academy of Sciences (CAS) developed an octahedra and tetrahedra composed flexible anionic framework for the stabilization of RE2+ in chalcogenides. The results published in The Innovation Materials (The Innov. Mater.,2025, 3, 100118.) with the title “Prismatic Crystal Field Stabilized Divalent Rare-Earth Chalcogenide REIIBII6CIII6QVI16 with Bifunctionality”, emphasizing the strategy effectiveness of anionic framework stabilizing RE2+ ions in chalcogenides.
In this work, based on the proposed anionic framework stabilizing RE2+ strategy, nine new RE2+ chalcogenides REIIBII6CIII6QVI16 (REII = La, Ce, Pr, Yb; BII = Mg, Mn; CIII = Al, Ga; QVI = S, Se) have been rationally designed, and synthesized in experiment by the high temperature solution method in sealed system. Amomg them PrMg6Ga6S16 is the first stable Pr2+ chalcogenide with evident luminescence emission properties. Moreover, the RE2+ is stabilized by a prismatic crystal field in the nine compound, which is different to the common octahedral crystal fields (Figure 1).
The nine compounds crystallize in the same NCS P6 space group, displaying bi-functional characterisitcs for luminescence emissions and NLO responses. For example, LaMg6Al6Se16 shows a wide visible lighe emission band under the exciation of ulraviolet source, as well as a a significant SHG response of 1.5 × AGS, and a high LIDT of 3 × AGS, demonstrating the multiple functionality of inorganic RE2+ compounds. The findings significantly advance the domain of RE chemistry, and give some new insights into the design of advanced RE2+ functional materials.
This work was done with South China Normal University and Jiangsu Normal University, and was financially supported by the Natural Science Foundation of the Xinjiang Uygur Autonomous Region, the Strategic Priority Research Program of the Chinese Academy of sciences and the National Natural Science Foundation of China.
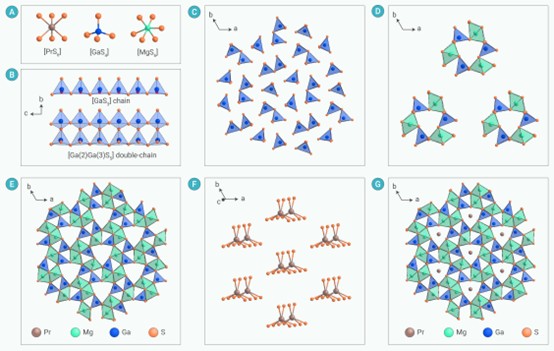
Figure 1. The crystal structure of PrMg6Ga6S16 (A) The coordination environments of Pr, Ga and Mg atoms; (B-C) The formed [GaS3] and [Ga(2)Ga(3)S5] chains viewed along a and c directions; (D) Windmill-like [Mg3Ga3S24] units; (E) [Mg6Ga6S16] framework; (F) The arrangement of [Pr2+S6] polyhedra in the ab plane; (G) The 3D crystal structure of PrMg6Ga6S16 viewed along c direction.
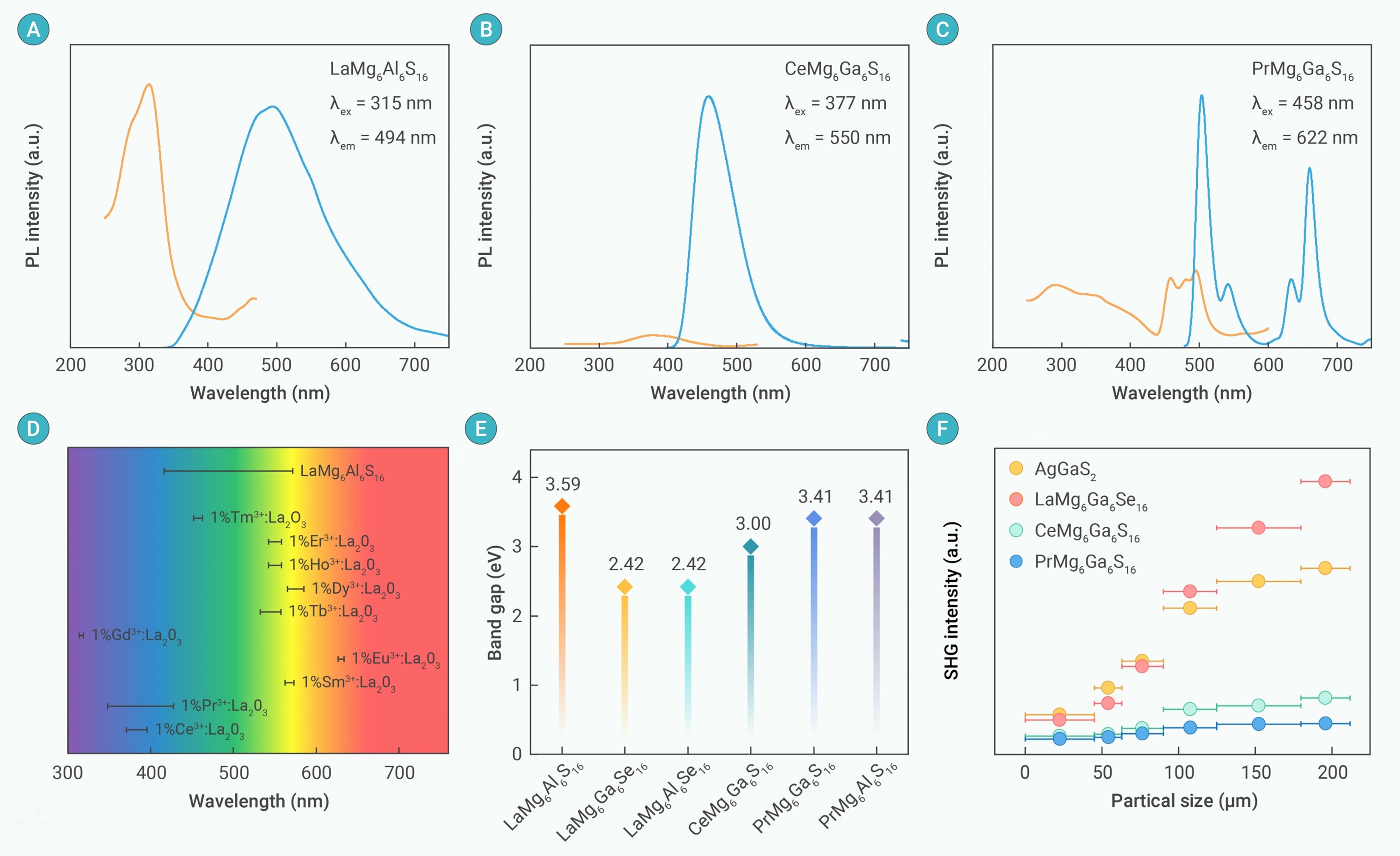
Figure 2. Optical properties (A-C) The PL excitation and emission spectra of LaMg6Al6S16 (A), CeMg6Ga6S16 (B) and PrMg6Ga6S16 (C); (D) The statistical analyses on the full width at half maximum (FWHM) of emission peak in LaMg6Al6S16 and typical RE3+-doped compounds; (E-F) The experimental optical band gaps (E) and the SHG intensities vs particle sizes (F) of REIIMg6CIII6QVI16.
附件下载:
 (86) 991-3838931
(86) 991-3838931 lhskj@ms.xjb.ac.cn
lhskj@ms.xjb.ac.cn (86)991-3838957
(86)991-3838957 40-1 Beijing Road
Urumqi, XinjiangChina
40-1 Beijing Road
Urumqi, XinjiangChina