Zero - Background Fluorescence Probe Enables Precise Hydrazine Detection
Editor: | Apr 23,2025
Hydrazine (N2H4) is a typical organic amine compound, which not only can be highly toxic and polluting, but also can spontaneously ignite when it reacts with strong oxidants, and will explode and decompose when exposed to air for a long time or at high temperature for a short time. The U.S. Environmental Protection Agency, the World Health Organization (WHO), and the International Agency for Research on Cancer (IARC) have classified N2H4 as a Class B2 hazardous substance, with a risk threshold of 0.01 mg/L or 312 mM recommended by the WHO. Hence, it is of great significance for national security, environmental protection and people’s safety to achieve rapid, visual and highly sensitive detection of N2H4. The fluorescence turn-on probe based on the excited-state intramolecular proton transfer (ESIPT) mechanism has higher sensitivity and can eliminate the background signal and interference caused by molecular collision quenching, which plays an irreplaceable role in the field of visual detection of hazardous chemicals. However, the proton donor substitution-release probe design strategy has certain limitations on the specificity of target analytes with similar structures and properties, and there is still a lack of systematic research on how the modulation of probe substituent properties affects the sensing performance of the non-fluorescent ESIPT-based probes. Hence, it is a challenging issue to propose a design strategy for the ESIPT-based probes from the perspective of modulating the substituent properties, and generating proton donors/acceptors after the probe reacts with the analyte, thereby improving its sensing performance.
To address this challenge, a research team led by Prof. DOU Xincun from the Xinjiang Technical Institute of Physics and Chemistry of the Chinese Academy of Sciences (CAS) has developed a design strategy of zero-background fluorescence probe to boost ultrasensitive and specific detection of hydrazine. Their findings, published in Analytical Chemistry, emphasize tuning the electron-accepting ability of para-substituent of the recognition site and the relative position of proton donor and recognition site to improve the reactivity of the probe towards N2H4 and the triggering ability of the ESIPT process.
In this work, researchers based on the nucleophilic attack characteristics of N2H4, a series of zero-background fluorescent probes (m-Br-OH-BDMN, m-CH3-OH-BDMN, OH-BDMN, Br-BDMN, p-Br-OH-BDMN) with dicyanoethylene as the recognition site were designed by regulating the electron-accepting ability of para-substituent (-Br>-H>-CH3) of the dicyanoethylene and the relative position (para or ortho) of the proton donor (-OH) and dicyanoethylene. It is found that a stronger electron-accepting capability could greatly improve the reactivity of the recognition site, and only when the hydroxyl group is in the ortho-substituent of the recognition site, the probe could react with N2H4 to generate hydrazone as a proton acceptor, producing the ESIPT process and the blue-green fluorescence emission. The probe m-Br-OH-BDMN with Br as the electron-accepting group has better detection performance for N2H4, with low limit of detection (LOD, 0.46 nM, 14.72 ng/L), fast response (1 s), superior selectivity even in the presence of 18 kinds of interferents including structural analogues of primary amines. Moreover, a silicon-based porous sensing material design strategy was proposed to efficiently adsorb and enrich target substances to improve molecular collision efficiency, which can effectively distinguish N2H4 from ethylenediamine solution and vapor within 5 s without interference from other volatile vapors. The presents non-fluorescent probe design strategy would provide new thoughts for the rational design of functional probes as well as high-performance sensing methodologies.
This work was supported by the National Natural Science Foundation of China, the Youth Innovation Promotion Association of the Chinese Academy of Sciences, the Tianshan Talents Plan.
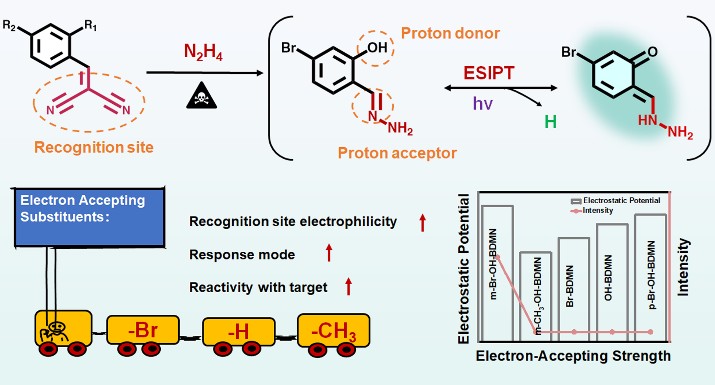
Figure: Schematic illustration of the designed strategy of the BDMN-based probe and the modulation of the electron-accepting strength of the para-substituent of the recognition site.
附件下载:
 (86) 991-3838931
(86) 991-3838931 lhskj@ms.xjb.ac.cn
lhskj@ms.xjb.ac.cn (86)991-3838957
(86)991-3838957 40-1 Beijing Road
Urumqi, XinjiangChina
40-1 Beijing Road
Urumqi, XinjiangChina