Copper-Deposited Basalt Fiber Fabric for Electrochemical CO2 Reduction to Ethanol with High Selectivity
Editor: | May 16,2025
The electrochemical CO2 reduction reaction (eCO2RR) to value-added multi-carbon (C2+) products, particularly ethanol, presents a compelling strategy for sustainable energy conversion and carbon neutrality. While copper (Cu) remains a benchmark electrocatalyst for C2+ product formation, inherent limitations concerning its density, mechanical robustness, susceptibility to corrosive environments, and the persistent challenge of achieving high C2+ selectivity and operational stability necessitate the exploration of innovative catalytic architectures. To address these critical shortcomings, this study introduces basalt fiber fabric (BFF), a cost-effective material derived from abundant volcanic rock, as a novel and high-performance support for Cu electrocatalysts. BFF was specifically chosen for its unique combination of properties: low density, exceptional mechanical resilience, superior chemical inertness, and inherent corrosion resistance, offering a compelling alternative to conventional catalyst supports. Furthermore, the Xinjiang province possesses significant natural reserves of basalt, making BFF a readily available and potentially low-cost material.
Capitalizing on these intrinsic attributes of BFF, a robust catalytic platform was engineered via a facile electroless Cu deposition by the research group of Prof. Abudukeremu Kadier and Prof. Peng-Cheng Ma at the Xinjiang Technical Institute of Physics and Chemistry (XTIPC) of the Chinese Academy of Sciences (CAS). This process yielded a uniformly deposited conductive material with a substantial Cu loading of 96.79 wt.%. The resultant Cu-deposited BFF exhibits a significantly reduced density (3.08±0.4 g/cm3) compared to bulk Cu (8.96 g/cm3), coupled with markedly enhanced mechanical properties (breaking forces of 3308±25 N in the warp direction and 665±20 N in the weft direction) and high electrical conductivity (4.81 × 10⁵ S/m pre-eCO2RR, minimally decreasing to 4.58 × 10⁵ S/m post-reaction).
Comprehensive electrochemical characterization in CO2-saturated potassium bicarbonate (KHCO3) electrolytes (0.1 M to 2.0 M) showed that the developed electrocatalyst achieved a current density of 25.93 mA/cm² with an exceptional Faradaic efficiency (FE) of 97.01% for ethanol production at an applied potential of -0.8 V vs reversible hydrogen electrode (RHE) in a conventional H-type cell. Mechanistic analysis revealed that while the catalyst predominantly favored the complex twelve-electron pathway for C2H5OH synthesis, minor competing two-electron (CO formation, 0.42% FE) and eight-electron (CH4 formation, 0.43% FE) processes were also observed, alongside a limited two-electron hydrogen evolution reaction (H2, 2.14% FE). The significantly higher FE for ethanol underscores the tailored surface properties and optimized reaction conditions that preferentially drive the multi-electron CO2 reduction pathway. Notably, increasing the electrolyte concentration to 1.5 M KHCO3 dramatically enhanced the catalytic performance, yielding an impressive current density of 184.51 mA/cm2 and a remarkable FE of 98.02% for ethanol, demonstrating unprecedented selectivity. Furthermore, the Cu-deposited BFF exhibited outstanding operational longevity, retaining 98.8% and 99.6% of its initial current density after continuous electrolysis for 100 h in 0.1 M and 1.5 M KHCO3 electrolytes, respectively. This high stability of over 100 h of continuous operation can be attributed to the inherent high mechanical strength and corrosion resistance of the BFF support, which effectively maintains the structural integrity and catalytic activity of the Cu. This research unequivocally establishes BFF, particularly leveraging the abundant resources of the Xinjiang province, as a novel, multifunctional, and scalable support material for the rational design of robust and high-performance Cu-based electrocatalysts for efficient CO2 conversion, offering a significant advancement towards sustainable chemistry and climate change mitigation.
The related research findings were recently published as a Research Article in Energy, entitled “Copper-deposited basalt fiber fabric for electrochemical CO2reduction to ethanol with 98% selectivity”.
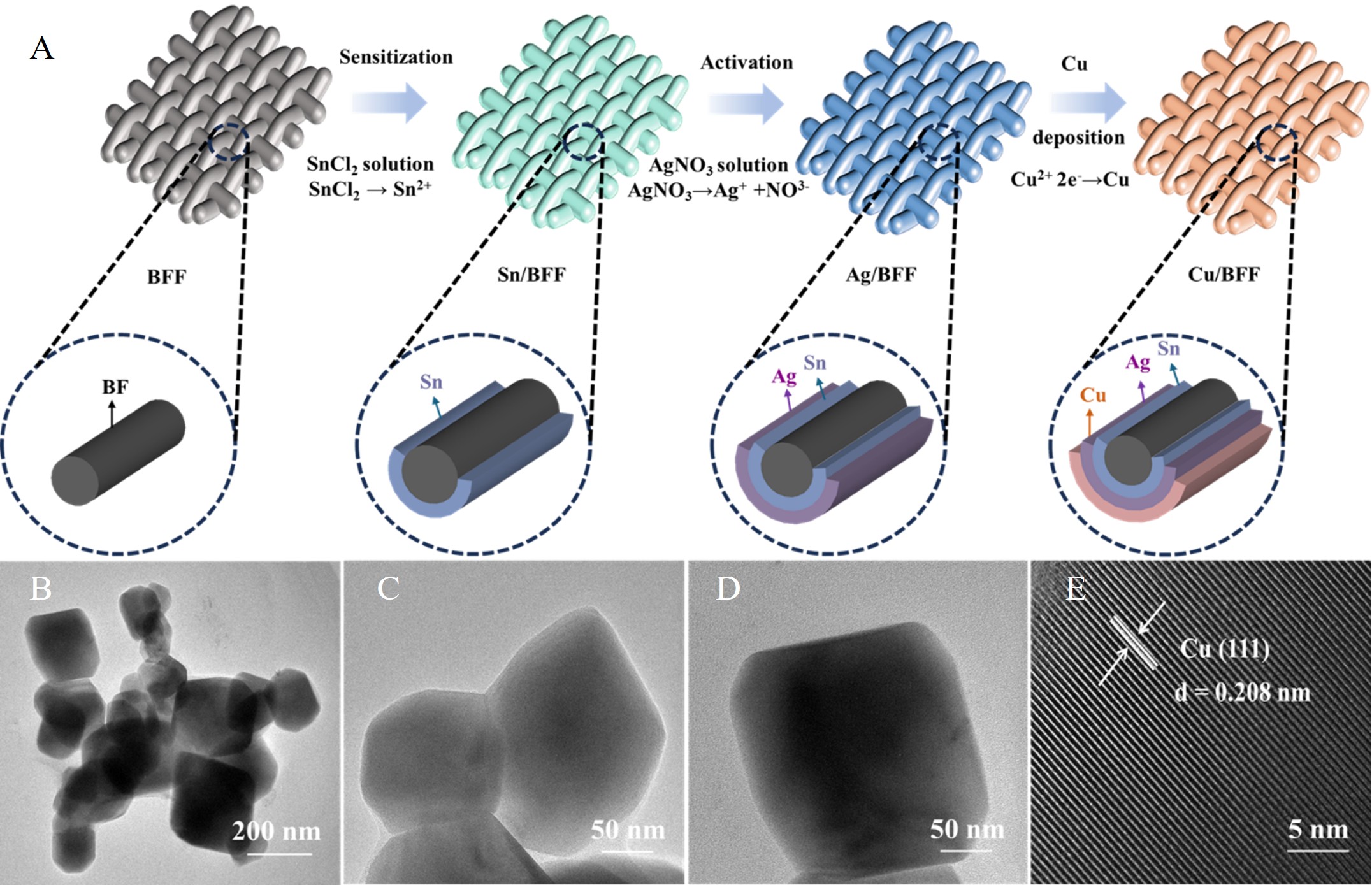
Fig. 1. Preparation and structural characterization of Cu-deposited BFF catalyst (A: Schematic illustration for preparing the Cu-deposited BFF; B-D: TEM images; E: HRTEM image of the Cu-deposited BFF, showing the crystalline lattice structure of the Cu layer).
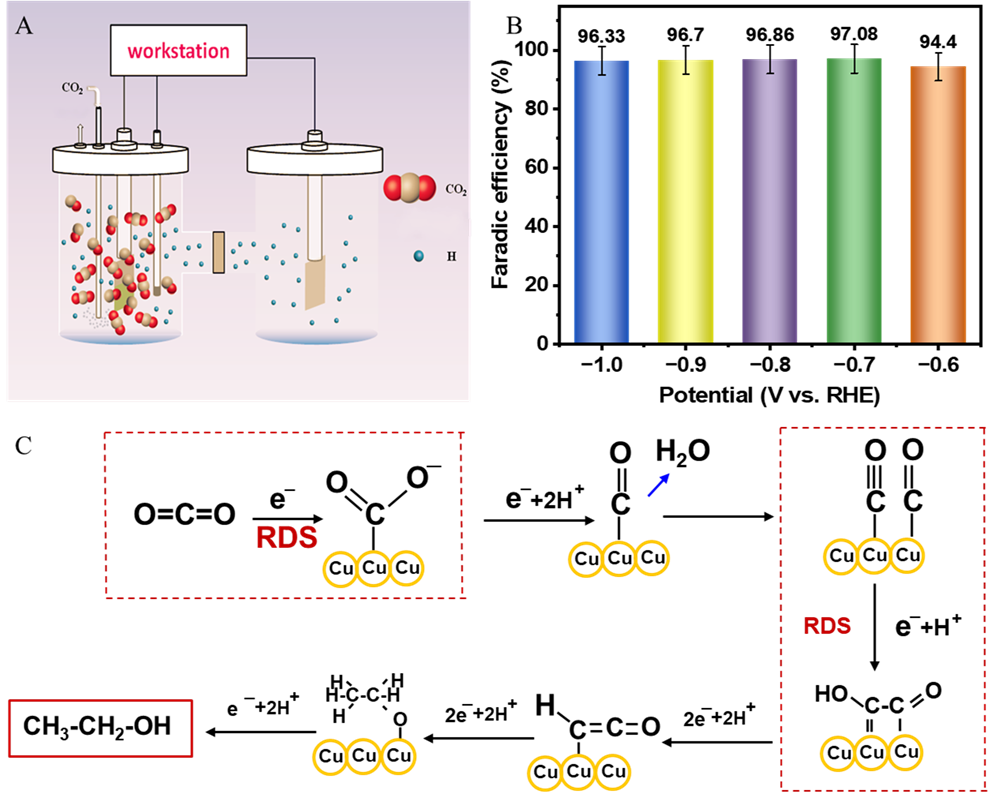
Fig. 2 Evaluation of eCO2RR on Cu-deposited BFF catalyst (A: Schematic diagram of the eCO2RR setup used in experiments; B: FE for C2H5OH production at different applied potentials ranging from -0.6 V to -1.0 V; C: Proposed reaction pathway for eCO2RR to C2H5OH on the Cu-deposited BFF surface).
附件下载:
 (86) 991-3838931
(86) 991-3838931 lhskj@ms.xjb.ac.cn
lhskj@ms.xjb.ac.cn (86)991-3838957
(86)991-3838957 40-1 Beijing Road
Urumqi, XinjiangChina
40-1 Beijing Road
Urumqi, XinjiangChina