Progress in the Exactly Matching the FRET Energy of the Functionalized Upconversion Nanoprobe to Boost the Sensing Performance toward Perchlorate
Editor: | Jun 22,2025
The accurate detection of trace chemical substances, including explosives, illegal drugs and nerve agents, is of great significance for ensuring national security and public safety. Xinjiang Key Laboratory of Trace Chemical Substances Sensing, Xinjiang Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, has dedicated extensive research to enhancing on-site detection performance through fundamental mechanism studies. The laboratory has developed a series of solutions to significantly boost the detection performance in aspects such as modulating fluorescent probe structures, constructing functional MOF sensing materials, and tuning amorphous nanomaterials (Angew. Chem. Int. Ed. 2025, 64, e202423576; Angew. Chem. Int. Ed. 2022, 61, e202203358; Adv. Mater. 2023, 35, 2300526; Adv. Mater. 2020, 32, 1907043; Adv. Funct. Mater. 2025, 35, 2425082; ACS Sensors 2025, 10, 1998; Anal. Chem. 2025, 97, 11669).
Perchlorate, a typical strong oxidizer commonly used in improvise explosives, solid propellants, aerospace equipment, fireworks, etc., however, there is still a big challenge for its chemical sensing in complex practical scenes. To address this challenge, Xinjiang Key Laboratory of Trace Chemical Substances Sensing has proposed a synergistic design strategy to construct an UCNP-FRET nanoprobe with accurate detection performance toward perchlorate by exactly matching the energy and the distance between donor UCNPs and acceptor ligand. Specially, a rhodamine B-based ligand with a thiourea group, 1-(2-(3’,6’-bis(diethylamino)-3-oxospiroisoindoline-1,9’-xanthen-2-yl)ethyl)thiourea (abbreviated as RhB-ligand), was designed to functionalize the core−shell NaYF4@NaYF4:Yb/Er UCNPs (abbreviated as RhB-UCNPs). It was found that the hydrogen bonding interactions between perchlorate and both thiourea and ethylamino groups of the RhB-ligand make the rhodamine B structure transform from a colorless spirocyclic state to a pink-color ring-opened state. Interestingly, this caused the ligand absorption spectrum exactly overlapping with the green emission spectrum of the UCNP donor, thus creating an effective FRET process from UCNPs to the RhB-ligand upon the detection of perchlorate. As a result, the perchlorate triggers the occurrence of the FRET in this UCNP-based nanoprobe and results in the obvious fluorescent change from green to red within 4 s, enabling an ultralow limit of detection (LOD) of 1.6 nM alongside a superior selectivity and anti-interference capability. Furthermore, the UCNP-FRET system exhibits exceptional detection performance by achieving ultra-accurate discrimination of trace perchlorate in an extremely complex situation containing multiple coexisting interferents, which strongly verifies the reliability of advancing nanoprobe utilization in practical scenarios. It is expected that the proposed nanoprobe design strategy would provide new insights for functional sensing material exploration for detecting perchlorate and other trace hazardous substances in complicated real-world scenarios.
This paper was published in Analytical Chemistry entitled as “Exactly Matching the FRET Energy of the Functionalized Upconversion Nanoprobe to Boost the Sensing Performance toward Perchlorate”. This work was supported by the National Natural Science Foundation of China, the Natural Science Foundation of Xinjiang, the Tianshan Innovation Team Plan, the Key Research Program of Frontier Sciences, the Central Guidance for Local Science and Technology Development Fund and the Youth Innovation Promotion Association of the Chinese Academy of Sciences.
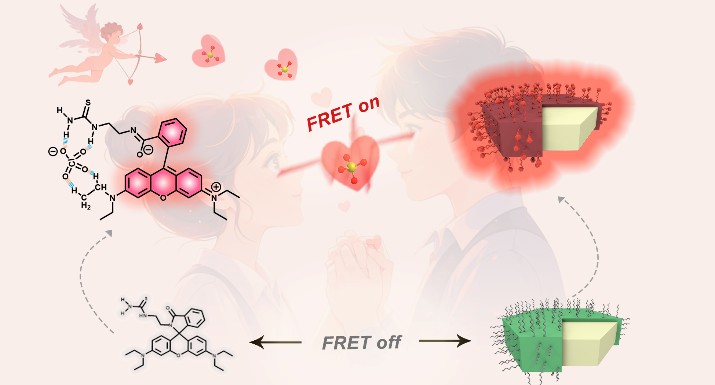
Figure: Schematic illustration of the FRET process in the RhB-UCNPs nanoprobe when detecting perchlorate
附件下载:
 (86) 991-3838931
(86) 991-3838931 lhskj@ms.xjb.ac.cn
lhskj@ms.xjb.ac.cn (86)991-3838957
(86)991-3838957 40-1 Beijing Road
Urumqi, XinjiangChina
40-1 Beijing Road
Urumqi, XinjiangChina