Research Progress in The Fluorescence Detection of Synthetic Cannabinoids
Editor: | Jul 07,2025
Synthetic cannabinoids are a class of artificially synthesized substances that mimic the psychoactive components in natural cannabis through chemical synthesis and belong to the category of new psychoactive substances. They act on the cannabinoid receptors (CB1 and CB2) in the human brain to produce hallucinogenic, excitatory, or sedative effects similar to those of natural cannabis. However, their chemical structures are completely different from those of natural cannabinoids, and they often have stronger toxicity and addictive potential. These substances are often disguised under names such as "spice" and "herbal incense", sprayed on herbs and tobacco leaves, or sold in the form of e-liquid and tablets, featuring high concealment and a high risk of abuse.
Currently, the detection methods for synthetic cannabinoids mainly include infrared spectroscopy, surface-enhanced Raman spectroscopy (SERS), nuclear magnetic resonance technology (NMR), gas chromatography-mass spectrometry (GC-MS), liquid chromatography-mass spectrometry (LC-MS), enzyme-linked immunosorbent assay, and electrochemical methods. Compared with the above-mentioned methods, fluorescence detection technology has the advantages of being intuitive, visual, highly sensitive, and accurate, and thus has a relative advantage and great development potential in on-site synthetic cannabinoid detection technology. For ADB-type and MDMB-type synthetic cannabinoids, which are currently abused more severely, fluorescent detection methods still have research gaps, and on-site rapid detection technologies are urgently needed.
Our team, starting from the molecular structure of ADB-type synthetic cannabinoids, has put forward an innovative probe design strategy. This strategy uses electronic effects (-Br, -H, -Ph) to enhance the cooperativity of noncovalent interactions between benzoxazole-based fluorescent probes and ADB-type synthetic cannabinoids, achieving specific recognition. A series of benzoxazole-based fluorescent probes (HBO-H, HBO-Br, and HBO-Ph) were constructed via acid-amine condensation. Studies have shown that regulating electronic effects can strengthen the noncovalent interactions and binding abilities between the probe and the target. Among these probes, HBO-Ph can recognize and bind ADB-type synthetic cannabinoids through π-π stacking, hydrophobic, and weak hydrogen-bonding interactions, producing a ratiometric fluorescence response from yellow-green to blue. Our team has uncovered the optical response mechanism. The ratiometric fluorescence emission of the probe-target complex originates from the suppression of excited-state intramolecular proton transfer (ESIPT) and a switch to intramolecular charge transfer (ICT)-based emission. HBO-Ph demonstrates the best detection performance for ADB-type synthetic cannabinoids, with a low detection limit of 24.3 nM, a rapid response time of less than 1 s, and high selectivity. Even in the presence of potential interferents like traditional drugs and new psychoactive substances, it remains unaffected. Furthermore, our team has developed a solid-liquid separation portable sensor based on this probe, enabling accurate detection of synthetic cannabinoids in complex samples such as e-liquid, tobacco leaves, and petals.
The work was published in Analytical Chemistry entitled as “Electronic Effect-Tuned Cooperativity of Multiple Noncovalent Interactions for Specific Detection of ADB-Type Synthetic Cannabinoids”. Zhang Xue, a master's student jointly trained by Xinjiang University, and Li Yudong, a special research assistant at the Xinjiang Technical Institute of Physics and Chemistry of the Chinese Academy of Sciences, are the co-first authors of the paper. The co-corresponding authors are Prof. Dou Xincun from the Xinjiang Technical Institute of Physics and Chemistry of the Chinese Academy of Sciences and Associate Professor Patima Nizamidin from the School of Chemistry, Xinjiang University.
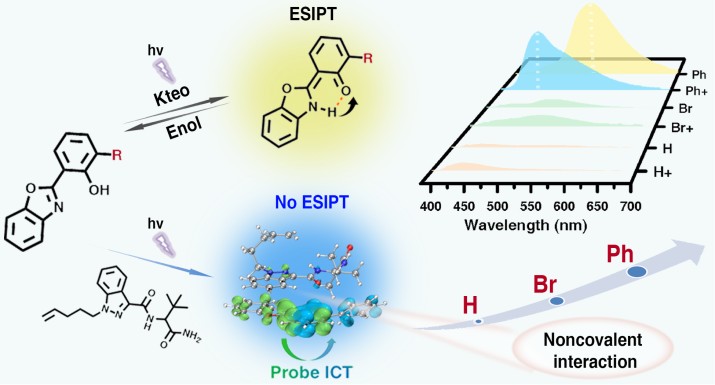
Figure: ESIPT-based probe design strategy and precise regulation of non-covalent interaction cooperativity via electronic effects (Imaged by the XTIPC)
To address the detection challenges of MDMB-type synthetic cannabinoids, which have low chemical activity and lack covalent reaction sites, the research team adopted an alternative approach. They designed and developed a fluorescent probe with two excited-state intramolecular proton transfer (ESIPT) sites, enabling specific recognition of MDMB-type synthetic cannabinoids. Combining theoretical computational analysis, the team systematically studied and revealed the mechanism of the probe for detecting MDMB-type synthetic cannabinoids: through multiple weak interactions between the probe and MDMB-type synthetic cannabinoid molecules, the ESIPT process of the probe itself was blocked, thereby triggering charge transfer within the probe, and ultimately achieving highly sensitive detection of MDMB-type synthetic cannabinoids. This probe exhibits excellent fluorescence sensing response performance towards MDMB-type synthetic cannabinoids. Specifically, the naked-eye fluorescence detection limit for MDMB-4en-PINACA is 0.20 mg/mL, the theoretical detection limit is 1.8×10⁻3 mg/mL, and the response time is only 1.2 s. In addition, the probe also has good selectivity and anti-interference ability, remaining unaffected by 20 potential co-existing interferents such as structural analogues, potential co-existing substances, and common drugs. To meet the practical needs of portable on-site detection of synthetic cannabinoids, the team developed a sensor device and successfully achieved on-site qualitative fluorescence detection of MDMB-4en-PINACA in six different drug carriers, including e-liquid, flower petals, tobacco leaves, tobacco shreds, chocolate, and popping candy. On this basis, the team further integrated deep-learning technology to achieve accurate identification and effective differentiation of six MDMB-type synthetic cannabinoids, providing strong technical support and guarantee for relevant detection work.
The relevant research, titled “Accurate Identification of MDMB-Type Synthetic Cannabinoids through Design of Dual Excited-State Intramolecular Proton Transfer Site Probe and Deep-Learning”, was published in Analytical Chemistry. Baoqing Chen, a jointly-trained master's student from Xinjiang Normal University, and Yudong Li, a special research assistant at the Xinjiang Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, are the co-first authors. Prof. Xincun Dou from the Xinjiang Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, and Prof. Ming Guan from the College of Chemistry and Chemical Engineering, Xinjiang Normal University, are the co-corresponding authors.
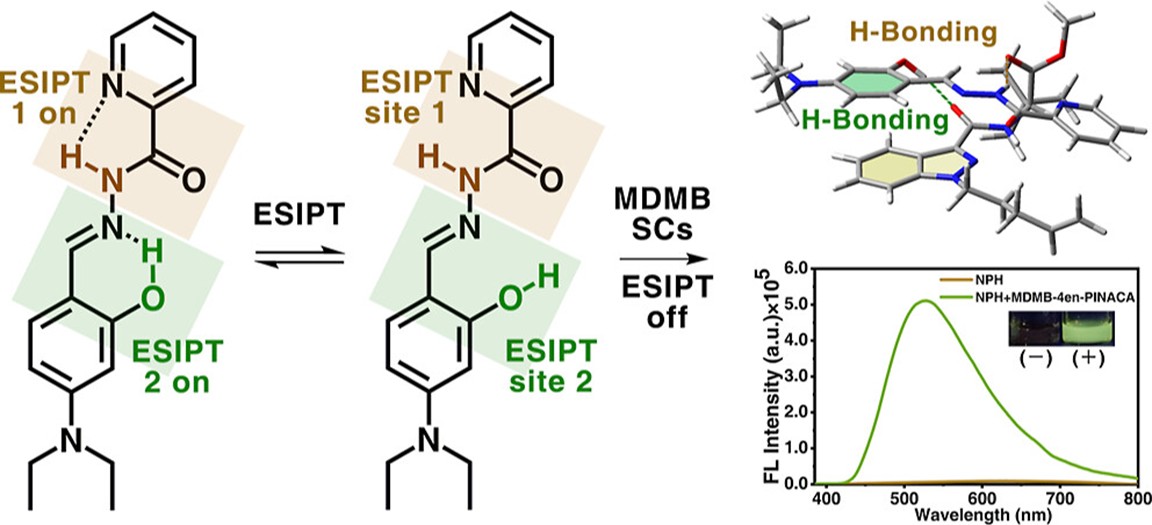
Figure: The detection of MDMB-type synthetic cannabinoids is achieved by using probes with dual ESIPT sites (Imaged by the XTIPC)
These two studies not only successfully fill the technical gap in the visual and rapid on-site detection of ADB-type and MDMB-type synthetic cannabinoids. The strategies proposed, namely “electronic effect-tuned multiple noncovalent interaction cooperativity” and “combination of dual ESIPT site probe design with deep-learning”, provide new ideas for probe design and methodological examples for detecting other new psychoactive substances with weak chemical activity and difficulty in capture. The developed portable sensor device is easy to operate and has a rapid response, which can meet the urgent need for rapid screening of concealed drugs in front-line anti-drug work.
附件下载:
 (86) 991-3838931
(86) 991-3838931 lhskj@ms.xjb.ac.cn
lhskj@ms.xjb.ac.cn (86)991-3838957
(86)991-3838957 40-1 Beijing Road
Urumqi, XinjiangChina
40-1 Beijing Road
Urumqi, XinjiangChina