Agriculture and industry development increased the load of potentially toxic compounds in the environment. Mercury (Hg) as one of themost harmful metals is widely used in the industries of pesticides,chlorine alkali chemistry, batteries, electronic devices and so on.Mercuric (Hg(II)) ions in water are highly reactive and possess a seriousthreat to both human health and ecological systems. Hg(II) ions canbe easily methylated, resulting in high level of toxicity even at a lowdosage. China’s National Sewage Comprehensive Emission Standard has suggested that 0.05 mg/L as the maximum allowable limit for Hg(II) in wastewater discharge. Therefore,developing cost-effective methods for removing Hg(II) ions from theaquatic environment with high efficiency is an urgent demand for securing public health. Recently, theEnvironmental Science and Technology Lab at XJIPC developed a novel polymer-based adsorbent thathyperbranchedpolyethylenimine functionalized carboxymethyl chitosan with asemi-interpenetrating networkcomposite(HPFC)was fabricated through a facile one-step crosslinking reaction.Notably, the as-prepared adsorbent demonstrated ultra-high adsorption capacity toward the removal of mercury ions. Upon treatment with 20mg-dosageof HPFC in an aqueous medium (20 mL), the concentration of mercury is decreased from 798.1 mg/L to 0.02 mg/L, which is below the national drainage standard of 0.05 mg/L for industrial wastewater in China. The maximum adsorption capacity is 1594 mg/g, which is higher than that of many other currently known natural polymer-based adsorbent. Furthermore, this adsorbent exhibits excellent selectivity for removing mercury ions, and the selectivity coefficient to mercury ions is 3-4 orders of magnitude higher than that to copper ions, cadmiumions and lead ions, respectively. Based on the measurements ofFTIR, XPS,and DFTcalculations,the adsorption mechanism was proposed. The removal of mercuryions by HPFC is mainly controlled by the chelation interaction between mercury ions and nitrogen groups and oxygengroups(i.e., amine, imine, and carboxyl groups). Significantly, HPFC has superior reusability and could be easily regenerated and reused multiple times. The developed HPFC adsorbent holds high potential in remediatingwater polluted with mercury ions. The related research results have been published in the international authoritative Journal of Chemical Engineering Journal and attracted extensive attention from peers. The research work was supported by the key projects of NSFC-Xinjiang joint fund. 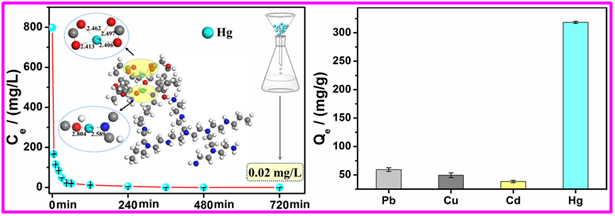 The adsorption of HPFC formercury ionsin aqueous solution |
