Niobate fluoride compounds have attracted much attention because of the versatile structures induced from various chemical substitutions at the cation or anion sites.
The research group headed by Prof. PAN Shilie from Xinjiang Technical Institute of Physics & Chemistry, Chinese Academy of Sciences, recently made a study in this respect. Single crystals of K2NaNbO2F4 were obtained from the system of KF–NaF–Nb2O5. And the structure of K2NaNbO2F4 was determined by single-crystal X-ray diffraction, and the crystal structure was discussed in detail. The compound is cubic, space group 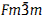 (No. 225), and the unit cell parameters are a=8.4726(4) Å, Z=4, Volume=608.21(5) Å3. Its structure can be described as three-dimensional frameworks formed of (0 1 0) [Na2Nb2O4F8] ∝N layers stacked along b axis. In the framework, niobium, oxygen, fluorine atoms form a rare [NbO2F4]3- anion group in an anhydrous solid state environment.
(No. 225), and the unit cell parameters are a=8.4726(4) Å, Z=4, Volume=608.21(5) Å3. Its structure can be described as three-dimensional frameworks formed of (0 1 0) [Na2Nb2O4F8] ∝N layers stacked along b axis. In the framework, niobium, oxygen, fluorine atoms form a rare [NbO2F4]3- anion group in an anhydrous solid state environment.
Meanwhile, the thermal stability and infrared spectrum of this compound were studied. The IR spectrum further confirmed the presence of NbO2 and NbF4 groups in the structure. The thermal analysis suggests that K2NaNbO2F4 is an incongruently melting compound.
The work has been supported by Chinese Academy of Sciences, the National Natural Science Foundation of China.The research results were published on Journal of Physics and Chemistry of Solids 73 (2012) 136–138.
Corresponding author:
PAN Shilie, Xinjiang Technical Institute of Physics and Chemistry, CAS.
Tel.: 86- 991- 3674558; E-mail: slpan@ms.xjb.ac.cn.
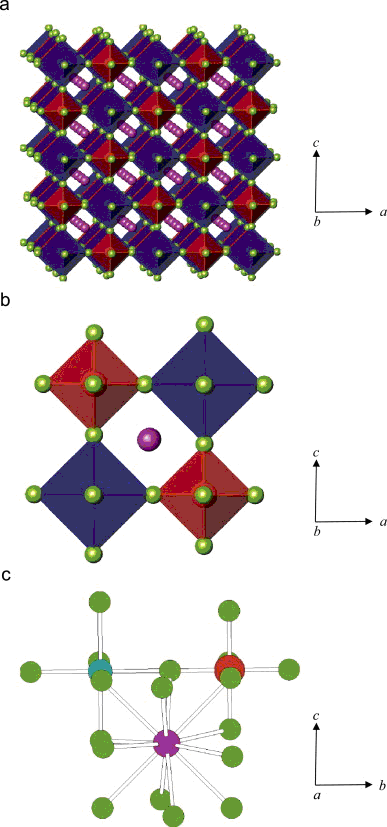
Fig. 1. Coordination polyhedra in K
2NaNbO
2F
4 (a), [Na
2Nb
2O
4F
8]
6- building units (b) and cations coordinate environments (c). The red octahedra are [NbO
2F
4]
3- anions and the blue octahedra are Na-centered. The green spheres are F or O atoms; the pink spheres are K; the blue spheres are Na, the red spheres are Nb atoms.(Image by LI Junjie)
