Nonlinear optical (NLO) materials have played an important role in laser science and technology. Some typical NLO materials, such as β-BaB2O4 (BBO), LiB3O5 (LBO), CsB3O5 (CBO), CsLiB6O10 (CLBO), and AgGaS2, have been used extensively. However, it is still a challenge to entirely meet the needs of the large second-harmonic generation (SHG) coefficient, wide transparent range, moderate laser damage threshold, and phase matchability for the increasingly expanding field.
Thus, understanding the correlation among the composition, structure, and NLO properties of crystals is still critical in exploring new SHG materials with better performance. As a remarkable source of the NLO material, borates have distinctive advantages, such as the large SHG, excellent UV transmittance, and high damage threshold value, etc.
Prof. Pan Shilie and his team from Xinjiang Technical Institute of Physics and Chemistry, CAS, synthesized a new noncentrosymmetric polyborate, Ba2[B6O9(OH)4], under a hydrothermal condition. The polyborate contains chiral layers constructed by two kinds of helical chains and also form new B18O42 circles based on B3O8 units [(3: Δ+2T)]. One kind of Ba atom locates in the cavity surrounded by the B11O11 ring within the anion layer, and the other kind of Ba atom inserts between two adjacent layers. UV–vis diffuse reflectance spectroscopy demonstrates that the compound has a cutoff edge below 190 nm and has a large second-harmonic generation (SHG) effect, which is approximately 3 times that of KH2PO4 (KDP) and is type-I phase matchable.
The result has been published on Inorganic Chemistry , 2012,51(3): 1852-1858. The paper is also archived at http://pubs.acs.org/doi/abs/10.1021/ic2021337.
The work was supported by the Main Direction Program of Knowledge Innovation of Chinese Academy of Sciences, the “National Natural Science Foundation of China”, the “One Hundred Talents Project Foundation Program” of the Chinese Academy of Sciences.
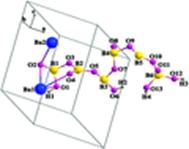
Figure 1. Asymmetric unit of Ba2[B6O9(OH)4]
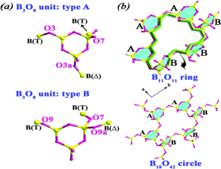
Figure 2. (a) Two kinds of spatial connections of the B3O8 units: type A and type B. (b) Perspective views of the B11O11 ring and B18O42 circle within the anion layer.
