Since Kirby’s pioneering work was reported in 1973, the hetero-Diels–Alder (HDA) reaction of acylnitroso compounds has been recognized as a useful tool in direct 1,4- functionalizations of conjugated dienes, and it provides easy access to a number of nitrogen- and oxygen-containing natural products.
Acylnitroso compounds are traditionally generated in situ by oxidizing hydroxamic acids using oxidants such as periodates, due to the high reactivity of acylnitroso species, these reactions are usually conducted in the presence of trapping agents to ensure formation of the desired products.However, the toxicity and inefficient economy of atom transfer in these oxidations processes means that there is a need to develop novel clean, catalytic methods. In recent years, mild protocols have been developed in which these oxidations are catalyzed by transition metals in conjunction witTBHP, H2O2, and dioxygen.
Researchers at the Xinjiang Technical Institute of Physics & Chemistry (XTIPC), CAS proposed a new strategy for the oxidation of hydroxamic acids and their Diels-Alder trapping.Dirhodium(II) caprolactamate [Rh2(cap)4] exhibits excellent oxidative reactivity toward hydroxamic acids when TBHP is the terminal oxidant; this catalytic combination in the presence of dienes provides HDA cycloadducts in high efficiency even at catalyst loadings as low as 0.1 mol% under mild reaction conditions (Scheme 1) . This catalytic is the first example for the dirhodium(II)-catalyzed oxidation of hydroxamic acid derivatives and significantly extends the utility of this unique catalyst.
The results were published on SYNLETT,2012, 23:1801–1804.
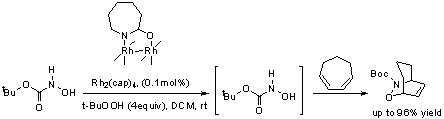
Scheme 1: in situ generation of acylnitroso dienophiles and their Diels–Alder trapping
