The design and synthesis of UV nonlinear optical (NLO) crystals appeared to be a more interesting and exciting research topic in material chemistry, for the best way to produce deep UV coherent light with solid state lasers is through cascaded frequency conversion using deep UV NLO crystals.Presently, a rational synthetic strategy widely used in this area is to link alkaline metal ions with borate functional building blocks, forming alkaline metal ions–borate system. It can provide building block with wide band–gapand alkaline metal–oxygen bond with no d–d electron transitions in this range promoting the discovery desired UV NLO crystals. It inspirits us to explore more borates in the systems to search for new functional materials.
Prof. PAN Shilie and his Ph.D. students from Xinjiang Technical Institute of Physics & Chemistry, Chinese Academy of Sciences (XTIPC), recently synthesized a new noncentrosymmetric polyborate, Li5Rb2B7O14, by solid–state reactions of stoichiometric amounts (Li:Rb:B=5:2:7) of Li2CO3, Rb2CO3 and H3BO3. And the single crystal of Li5Rb2B7O14 has been synthesized through spontaneous crystallization. A mixture of Li2CO3 (98.0%), Rb2CO3 (99.5 %), and H3BO3 (99.5%) was placed into a platinum crucible in the molar ratio corresponding to Li:Rb:B=6:5.5:12. Then the crucible was put into the furnace and heated to 750 °C, kept at this temperature for 24 h, then cooled at 5 °C/min to 570 °C and finally quenched to room temperature at a rate of 10 °C/min. Colorless block crystals were separated from the crucible for structural characterization.The crystal structure of Li5Rb2B7O14 was investigated by single–crystal X–ray diffraction on a Bruker SMART APEX II CCD diffractometer using monochromatic Mo Kα radiation (λ = 0.71073 Å) at 296(2) K and integrated with the SAINT program. The infinite three–dimensional structure of Li5Rb2B7O14 constructs from bridged LiOn (n = 4, 5, 6) and RbO10 polyhedra with two kinds of one–dimensional B–O chain fundamental building blocks through their vertex oxygen atoms.
Thermal analyses were carried out on NETZSCH STA 449 F3 Jupiter instrument at a temperature range of 30–850 °C with a heating rate of 10 ºC·min–1 in an atmosphere of flowing N2. The DSC curve of Li5Rb2B7O14 shows one endothermic peak at 637 °C on the heating curve, which tentatively suggests that Li5Rb2B7O14 melts congruently at 637 °C. UV–VIS–NIR diffuse–reflectance data were collected with a SolidSpec–3700DUV spectrophotometer with fluororesin as the standard, in the wavelength range from 190 to 2600 nm. The powder sample was obtained by spontaneous crystallization from a stoichiometric ratio melt. It exhibits a short–wavelength absorption onset at 190 nm. The feature of a short UV cut off edge is favorable in practical applications. The electronic property calculations were performed using the first principles plane-wave pseudopotential method implemented in the CASTEP package. The calculated band structures and the density of states of Li5Rb2B7O14 suggest that its indirect energy gap is 4.247 eV. Further investigation for the growth of large crystals and related physical property studies is under way.
The work has been supported by the National Natural Science Foundation of China,the “One Hundred Talents” program of Chinese Academy of Sciences. The article was published in CrystEngComm.
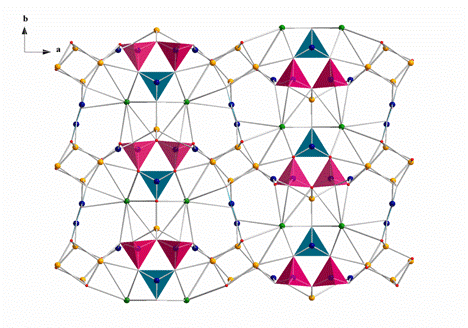
View of the structure of Li5Rb2B7O14 and cations coordination environments down c axis(orange, Li; green, Rb; blue, B; red, O; purple, BO4 group; light blue, BO3 group). (Image by XTIPC)
