Intensive research on borophosphates has been carried throughout these years due to their rich structural chemistry and many potential applications. Becuse the B atoms reveal either triangular or tetrahedral coordination modes, the BO3 and BO4 as well as PO4 groups are interconnected via common corners to form complex anionic structures, such as isolated species, oligomers, rings, chains, layers and frameworks. Therefore, the considerable variety in chemical structure of borophosphates provides a great deal objects for exploring novel compounds. In general, the P-O-P connections are extremely rare among borophosphates with the possible explanations found in Pauling’s fourth rule. However, novel borophosphate compounds are still discovered with time.
 |
|
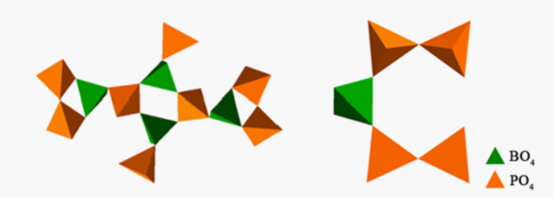
[B4P8O30] unit (left) and [B(P2O7)2] unit (right) give further examples of borophosphate frameworks with a P-O-P connection |
Prof. PAN Shilie and his Ph.D. students from Xinjiang Technical Institute of Physics & Chemistry, Chinese Academy of Sciences (XTIPC), have synthesized four new anhydrous mixed alkali-metal borophosphates, Li
2Cs
2B
2P
4O
15, LiK
2BP
2O
8, Li
3K
2BP
4O
14 and Li
3Rb
2BP
4O
14, through solid-state method. The four compounds are structurally related and constituted by anionic group layers and metallic polyhedra. The differences among them are mainly caused by the different fundamental building units (FBU). Li
2Cs
2B
2P
4O
15 contains a novel anionic partial structure [B
4P
8O
30], which provides further evidence that the P-O-P linkers exist in borophosphates. LiK
2BP
2O
8 contains very rare [BP
2O
8]
n3n- layers with [B
2P
4O
16] as its FBU. Li
3K
2BP
4O
14 and Li
3Rb
2BP
4O
14 are isotypic, and they have [B(P
2O
7)
2] FBU with another kind of P - O - P linkers.
The four compounds represent the first example of mixed alkali-metal anhydrous borophosphates. Besides, except for LiK2BP2O8, the other three compounds express two unusual anionic partial structures, which provide further examples of the P-O-P connections and expand the structural chemistry of borophosphates.
The research paper has been published in Chemistry - A European Journal ,2012, 18: 12046–12051.
