Polycyclic aromatic hydrocarbons (PAHs), mainly produced via fossil fuel deposit and incomplete combustion, have been primarily detected in naturally hydrophobic phases such as soil and sediment. PAHs are also found in cooked foods, such as grilling or barbecuing, and in smoked fish. As a pollutant, they have been attracted increasing attention because some compounds have been identified as carcinogenic,mutagenic and teratogenic. Among them, phenanthrene is one of the most widespread PAHs with three fused benzene rings, and it is often used as a model compound to study the transformation and fate of PAHs in contaminated soils.
Researchers from Xinjiang Technical Institute of Physics & Chemistry, Chinese Academy of Sciences(XTIPC), found that the soil components (such as humic acid and natural minerals) significantly impact the stability and fate of PAHs, for example, their photolysis processes in the soil environment.
Researchers used phenanthrene as a model to explore the roles played by three soil organic matter (SOM) fractions, including dissolved organic matter (DOM), humic acid (HA), and fulvic acid (FA), in its photodegradation with assistance of Fe(III)-smectite under visible-light. After observation, researchers found slight decrease in phenanthrene photodegradation rate in the presence of DOM, it can be explained in terms of oxidative-radical competition between DOM and target phenanthrene molecules due to the high electron–donor capacity of phenolic moieties in DOM. Besides,a critic content is observed with FA (0.70 mg/g) and HA (0.65 mg/g). Before reaching the critic content, the removal of phenanthrene is accelerated; while after that, the photodegradation rate is suppressed (Fig.5). In general, SOM fractions on clay surface exert various effects including photosensitization, complex formation, quenching radicals, and block on reactive sites. These effects are often associated with each other in the natural soil environment.
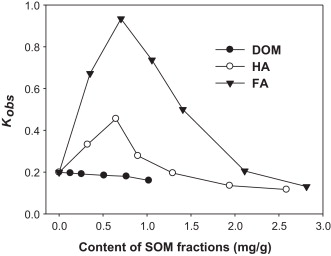
Effect of the SOM fractions content on phenanthrene photodegradation
The result has been published in Journal of Hazardous Materials (Volumes 256–257, 15 July 2013, pp 16–23).
The work was supported by the National Natural Science Foundation of China, “Hundred Talents Program” of CAS, the “Cross-Cooperation Program for Creative Research Teams” of CAS, etc.
