In recent years, tantalates have been extensively studied because of their high photocatalytic activities for splitting water into H2. However, solid state methods are always used for tantalates synthesis at high temperature of around 1000 °C over long periods, which may result in large grain growth (thus reduction in surface areas), leading to a decrease in photocatalytic activity.
A novel approach to synthesize K1.9Na0.1Ta2O6·2H2O (KNTO) nanocrystals with controllable crystalline facets was taken by a team of researchers lead by WANG Chuanyi from Xinjiang Technical Institute of Physics & Chemistry, Chinese Academy of Sciences (XTIPC). Researchers employed two-step synthetic approach, namely, molten salt in combination with hydrothermal processing. This strategy is highlighted by the fact that the shape and facet controlling of KNTO is achieved by simply adjusting the concentration of KOH addition in the hydrothermal reaction. Besides, photocatalytic activity of the synthesized KNTO for H2 evolution from CH3OH/H2O solution was successfully tuned through the specific control of exposed facets.
Researchers prepared selectively two distinct KNTO nanocrystals, {111}-terminated octahedra and {101}-dominated nanosheets, by varying the amount of KOH added in the hydrothermal process. They found the as-obtained crystals show high activity in photocatalytic H2 evolution from CH3OH/H2O solution, and their performances mainly depend on the exposed facets. Based on test results, the rate of H2 evolution under UV–light irradiation is about 4.4 μmol h–1 for the KNTO nanooctahedra, which is 3 times higher than that of KNTO nanosheets dominantly exposed with {101} facets. The facet-dependent activities can be attributed to a cooperative effect of surface atomic structure (the density of undercoordinated Ta atoms) and electronic structure (the position of conduction band minimum).
This is the first report for the facet controllable synthesis of tantalate materials with highly regular shapes. The present approach is anticipated as a general strategy for controllable synthesis of tantalate materials and also provides feasibility for rational design and activity optimization of shape-controlled photocatalysts for H2 production.
The results of this study have been published in ACS Appl. Mater. Interfaces, 2013, 5 (20), pp 10260–10265.
This work was supported bythe National Nature Science Foundation of China,the “Hundred Talent Program” of CAS,the “Cross-Cooperation Program for Creative Research Teams” of CAS, the Excellent Youth Foundation of Xinjiang,etc.
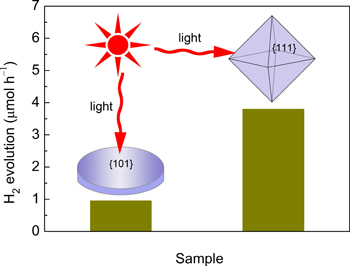
Facet–dependent photocatalytic activity for H2 production of the KNTO nanocrystals with predominant {111} and {101} facet (Image by XTIPC)
