Borosilicates have attracted much attention due to their many potential applications and abundant new structural types from the combination of the flexible coordination geometries for both B and Si atoms. As is known to all, inorganic compounds with polar coordination units are more likely to display NCS structures and exhibit good SHG properties. It has been proved to be fruitful in obtaining polar structures by inclusion of an asymmetric salt unit as well as monatomic halide anions as a template.
In order to design and discover new NLO crystals, approaches by the inclusion of the acentric salt units as polar template are often adopted. Recently, Professor PAN Shilie and his team from Xinjiang Technical Institute of Physics & Chemistry, Chinese Academy of Sciences (XTIPC), designed and synthesized two new salt-inclusion compounds, Ba4(BO3)3(SiO4)·Ba3X (X = Cl, Br), which are based on the combination of two different anionic groups (SiO4 and BO3) and the utility of XBa6 (X = Cl, Br) polar template (Fig.1). It is worth to note that they are the first alkaline-earth metal borosilicate halides as nonlinear optical (NLO) materials. They have SHG responses similar to that of KH2PO4 (KDP) and are type-I phase-matchable. In addition, they melt congruently and exhibit a wide transparent region with the UV cut-off edge below 190 nm. These properties make Ba4(BO3)3(SiO4)·Ba3X potential deep UV NLO materials.
Researchers analyzed about the “Polar Templating” Effect of XBa6 salt inclusion units, the results indicate that the material may crystallize in an NCS structure when the distorted direction of the acentric XBa6 SBU are in a parallel manner so that producing macroscopic acentricity.
The research results have been published in the J. Mater. Chem. C, 2014.
This work is supported by the “National Natural Science Foundation of China”, the Foundation of the Youth Innovation Promotion Association of CAS, 973 Program of China, etc.
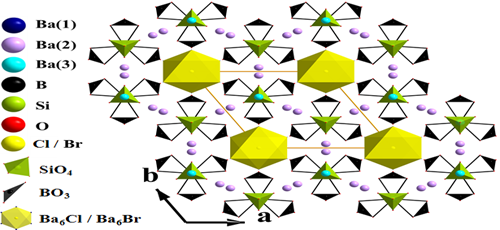
Fig.1 The 3D framework of Ba4(BO3)3(SiO4)·Ba3X(X = Cl, Br) (Image by XTIPC).
