Recently, photocata lysis has attracted much attention due to its great potential in tackling energy and environmental challenges. Among all photocatalysts, TiO2-based catalysts appear as the most promising candidates for their high photocatalytic activities, environmental benign nature and large abundance. Unfortunately, the large band gap (∼3.2 eV) of prestine TiO2 makes it only photoactive in UV light, which limits its efficiency powered by sunlight, as UV light only accounts for 5% energy of the entire solar spectrum while 43% solar energy resides in visible region.
The research team led by Prof. WANG Chuanyi at Xinjiang Technical Institute of Physics & Chemistry (XTIPC),employed a facile molten salt synthesis (MSS) methodology to achieve size-controlled BTO nanosheets with predominant exposure of {0 0 1} facets by simply varying the mole ratio (M) of salts (NaCl and KCl) to Bi4Ti3O12 (BTO) from 4 to 60.
Researchers found the size of the BTO-M samples decreases with the increase f the M value, which enhances the BET surface area of the particles for improved the photocatalytic activities. Meanwhile, BTO single crystals exhibit spontaneous polarization (Ps) of 4 μC cm−2 along the c-axis. Thus, the formed internal electric field along [0 0 1] direction can promote the separation of photoinduced charges. This effect is proven by tracking the surface-potential change of the BTO-50 sample in dark and under visible-light irradiation using kelvin probe force microscopy (KPFM) analysis.
It is the first time to investigate how the ferroelectric nature of BTO affects the photocatalytic activities. Besides, the nanosheet structure also shortens the separation distance of photoinduced electron–hole pairs along [0 0 1] direction, as evidenced by the improved photocatalytic kinetics by a factor of about 8 times higher than that of BTO-0 sample prepared through a traditional solid-state reaction method.
The results have been published in Applied Catalysis B: Environmental,Volumes 156-157, Pages 35-43.
The work was supported by the National Nature Science Foundation of China, the “Hundred Talent Program” of Chinese Academy of Sciences, the CAS/SAFEA International Partnership Program for Creative Research Teams, etc.
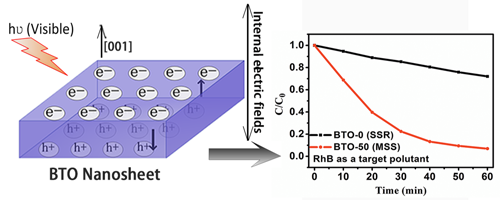
Fig. Model showing thedirection of the internal electric field in BTO-M samples (left). Degradation kinetics of RhB at the BTO samples under visible-light irradiation (right).
