Chlorophenols (CPs) represent one important class of organic pollutants, for which the stable chlorine-carbon (C-Cl) bond sustains the toxicity and carcinogenicity. Cleavage of the C-Cl bond remains a critical step of the degradation process of CPs, usually occurring at the first stage of reactions. It would be of great importance to develop a highly effective catalyst that will enable fast dechlorination of CPs under mild reaction conditions.
Researchers from Xinjiang Technical Institute of Physics & Chemistry of Chinese Academy of Sciences fabricated one type of borate nonlinear optical material, K3B6O10Br (KBB), which demonstrated excellent catalytic activity in UV-induced (λ > 254 nm) light irradiation. The obtained dechlorination efficiency was 90 times higher than that of commercial P-25 TiO2 catalyst under same reaction conditions. The noncentrosymmetric structure of KBB produced an intrinsic large polarization effect as evidenced by Kelvin probe force microscopy. The result showed that the spontaneous polarization arising from the displacement of the center of the positive and negative charges was responsible for providing a driving force for the separation of photogenerated electrons and holes and mitigating the effect of recombination as confirmed by Kelvin probe force microscopy.
A spontaneous polarization with directions pointing from the bulk to the surface usually produces a positive charge on the surface (C+ domain), and the polarization pointing away from the surface to the bulk will generate a negative charge (C− domain). The spontaneous polarization can be screened by free electrons and holes, and/or by ions or molecules adsorbed on the surface from forming a Stern layer. This accumulation of free electrons on the C+ surface and holes on the C− surface leads to downward and upward band bending, respectively. The internal dipolar field creates charged surfaces that cause photogenerated charge carriers to move in opposite direction which separates electron-hole pairs, leading to oxidation and reduction products formation at different locations. The findings were published in Chemistry of Materials.
Based on the previous work, the researchers broadened the absorption edge of the materials. They found that the Vanadium appeared to be one of the best alternatives to improve visible light-driven photoactivity since it played an important role in extending optical absorption region and the high separation efficiency of electron-hole pairs. Researchers combined the borate with the vanadium to find out a simple vandate-boron semiconductor, Na3VO2B6O11 (NVB), which was also a kind of Nonlinear Optical (NLO) material. The UV-vis diffuse reflectance spectrum further confirmed that the NVB particles could absorb photons in wavelength up to 419 nm. The result showed that this material could function as an effective photocatalyst for dechlorination under UV-visible light irradiation (λ > 320 nm). The findings were published in Journal of Materials Chemistry A.
These results provide inspiration for designing efficient photocatalysts for nonlinear materials.
The work was supported by the National Natural Science Foundation of China, Western Light of Chinese Academy of Sciences, Xinjiang International Science & Technology Cooperation Program, etc.
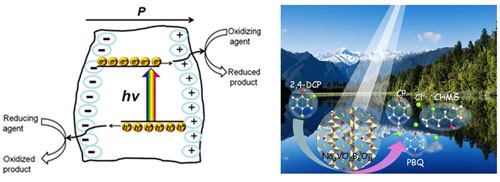
Figure: Schematic illustration of a non-centrosymmetry structured photocatalyst and the course of photo-dechlorination of 2,4-DCP. (Image by XTIPC)
Contact:
Prof. WANG Chuanyi
E-mail: cywang@ms.xjb.ac.cn
Xinjiang Technical Institute of Physics & Chemistry,CAS
