Fuel cells and metal-air battery are considered as promising clean and high efficient energy systems to convert chemical energy directly into electrical energy. Technical limitation associated with the application, in particular the cathode catalyst for low oxygen reduction reaction (ORR) activity, has proven to be a complicated bottleneck to overcome.
As one of the most promising non-precious metal catalysts with superior methanol tolerance, Fe-N-C exhibits super ORR electrocatalytic activity. However, the correlation between the type and number of Fe-Nx active sites versus the ORR activity is still ambiguous. And it is still a great challenge to produce Fe-Nx-based materials with a single structure to better understand the catalytic active sites for ORR.
A research group led by Prof. HU Guangzhi at Xinjiang Technical Institute of Physics and Chemistry of Chinese Academy of Sciences collaborating with Prof. Thomas Wågberg’s group at Umeå University and Guo Shaojun’s group at Peking University achieved the catalyst synthesis of atomically dispersed FeN2 moieties onto the surface of N-doped ordered mesoporous carbon for boosting ORR electrocatalysis via intriguing template casting strategy.
The high-angle annular dark field-scanning transmission electron microscopy (HAADF-STEM) disclosed that the elements C, N and Fe were homogeneously distributed on the surface of materials. 57Fe Mössbauer spectroscopy confirmed that Fe were only coordinated by N atoms, and extended X-ray absorption fine structure (EXAFS) at Fe k-edge X-ray absorption spectroscopy revealed that the the coordination number of N is 2.0.
The density of FeN2 is calculated by X-ray photoelectron spectroscopy (XPS). Due to a lower interaction with *O2 and *OH intermediates and enhanced electron transport, the abundant atomic dispersion of FeN2 on mesoporous carbon showed better activity and selectivity toward four-electron reduction of O2 than the commercial Pt/C catalyst in alkaline medium.
The well-defined structure prompts researchers to understand the nature of the catalytic active sites, and to demonstrate that the catalyst activity is linearly proportional to the concentration of FeN2 sites. Deduced from Butler-Volmer formulation, a kinetic model was established to predict the ORR activity of these single-atom dispersed catalysts. The results are published on Nano Energy.
Moreover, researchers introduced sublimed sulfur as S precursor during the synthesis and it was finally doped into the carbon skeleton to form thiophene-like structures (C-S-C). The synergistic effect between C-S-C and Fe-N-C sites reduces the electron localization around the Fe center, improves the interaction with oxygenated species, and therefore boosts its ORR activity in acid solution. The results were published in Angewandte Chemie International Editon.
The work was supported by National Natural Science Foundation, Xinjiang International Science and Technology Cooperation Project, National Key Research and Development Program of China, Thousand Talent Program.
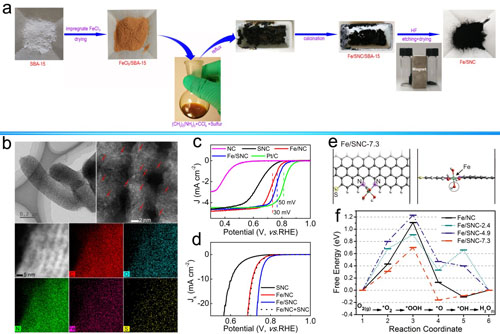
Figure: (a) Schematic synthesis procedure illustration, (b) Microzone characterization, (c-e) electrochemical activity test analysis and (f) Free energy diagram for the Fe/SNC.(Image by XTIPC)
Contact:
Prof. HU Guangzhi
E-mail:guangzhihu@ms.xjb.ac.cn
Xinjiang Technical Institute of Physics & Chemistry, CAS
